Multimodal Raman spectroscopy and optical coherence tomography for biomedical analysis
- PMID: 36308009
- PMCID: PMC10082563
- DOI: 10.1002/jbio.202200231
Multimodal Raman spectroscopy and optical coherence tomography for biomedical analysis
Abstract
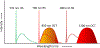
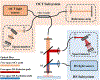
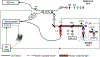
Optical techniques hold great potential to detect and monitor disease states as they are a fast, non-invasive toolkit. Raman spectroscopy (RS) in particular is a powerful label-free method capable of quantifying the biomolecular content of tissues. Still, spontaneous Raman scattering lacks information about tissue morphology due to its inability to rapidly assess a large field of view. Optical Coherence Tomography (OCT) is an interferometric optical method capable of fast, depth-resolved imaging of tissue morphology, but lacks detailed molecular contrast. In many cases, pairing label-free techniques into multimodal systems allows for a more diverse field of applications. Integrating RS and OCT into a single instrument allows for both structural imaging and biochemical interrogation of tissues and therefore offers a more comprehensive means for clinical diagnosis. This review summarizes the efforts made to date toward combining spontaneous RS-OCT instrumentation for biomedical analysis, including insights into primary design considerations and data interpretation.
Keywords: Raman spectroscopy; biomedical; label-free; multimodal; optical coherence tomography.
© 2022 Wiley-VCH GmbH.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
Figures





Similar articles
-
Depth-resolved multimodal imaging: Wavelength modulated spatially offset Raman spectroscopy with optical coherence tomography.J Biophotonics. 2018 Jan;11(1). doi: 10.1002/jbio.201700129. Epub 2017 Aug 17. J Biophotonics. 2018. PMID: 28703472
-
Morpho-molecular ex vivo detection and grading of non-muscle-invasive bladder cancer using forward imaging probe based multimodal optical coherence tomography and Raman spectroscopy.Analyst. 2020 Feb 17;145(4):1445-1456. doi: 10.1039/c9an01911a. Analyst. 2020. PMID: 31867582
-
Multimodal Scanning Microscope Combining Optical Coherence Tomography, Raman Spectroscopy and Fluorescence Lifetime Microscopy for Mesoscale Label-Free Imaging of Tissue.Anal Chem. 2021 Aug 24;93(33):11479-11487. doi: 10.1021/acs.analchem.1c01637. Epub 2021 Aug 11. Anal Chem. 2021. PMID: 34380310
-
Looking for a perfect match: multimodal combinations of Raman spectroscopy for biomedical applications.J Biomed Opt. 2021 Aug;26(8):080601. doi: 10.1117/1.JBO.26.8.080601. J Biomed Opt. 2021. PMID: 34387049 Free PMC article. Review.
-
Raman Plus X: Biomedical Applications of Multimodal Raman Spectroscopy.Sensors (Basel). 2017 Jul 7;17(7):1592. doi: 10.3390/s17071592. Sensors (Basel). 2017. PMID: 28686212 Free PMC article. Review.
Cited by
-
Towards Optical Biopsy in Glioma Surgery.Int J Mol Sci. 2025 May 9;26(10):4554. doi: 10.3390/ijms26104554. Int J Mol Sci. 2025. PMID: 40429698 Free PMC article. Review.
-
Single-fiber probes for combined sensing and imaging in biological tissue: recent developments and prospects.Biomed Opt Express. 2024 Mar 15;15(4):2392-2405. doi: 10.1364/BOE.517920. eCollection 2024 Apr 1. Biomed Opt Express. 2024. PMID: 38633092 Free PMC article. Review.
-
From Vibrations to Visions: Raman Spectroscopy's Impact on Skin Cancer Diagnostics.J Clin Med. 2023 Nov 30;12(23):7428. doi: 10.3390/jcm12237428. J Clin Med. 2023. PMID: 38068480 Free PMC article. Review.
-
Optical theranostics in ischemic heart disease: from molecular insights to clinical translation.Theranostics. 2025 Jun 9;15(14):6789-6817. doi: 10.7150/thno.114307. eCollection 2025. Theranostics. 2025. PMID: 40585968 Free PMC article. Review.
-
Label-free biomedical optical imaging.Nat Photonics. 2023 Dec;17(12):1031-1041. doi: 10.1038/s41566-023-01299-6. Epub 2023 Nov 16. Nat Photonics. 2023. PMID: 38523771 Free PMC article.
References
-
- Wan Q-S, Wang T, Zhang K-H, Tumor Biol. 2017, 39, 1010428317717984. - PubMed
-
- Majumder SK, Keller MD, Boulos FI, Kelley MC, Mahadevan-Jansen A, J. Biomed. Opt 2008, 13, 054009. - PubMed
-
- Stone N, Kendall C, Shepherd N, Crow P, Barr H, Raman Spectrosc J. 2002, 33, 564.
-
- H H. Mantsch, Infrared and Raman Spectroscopy of Biological Materials, CRC Press, Boca Raton, FL: 2000, p. 4.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

