Swelling-Dependent Shape-Based Transformation of a Human Mesenchymal Stromal Cells-Laden 4D Bioprinted Construct for Cartilage Tissue Engineering
- PMID: 36308047
- PMCID: PMC11468569
- DOI: 10.1002/adhm.202201891
Swelling-Dependent Shape-Based Transformation of a Human Mesenchymal Stromal Cells-Laden 4D Bioprinted Construct for Cartilage Tissue Engineering
Abstract
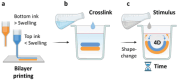
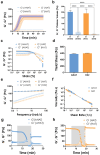
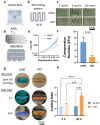
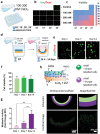
3D bioprinting is usually implemented on flat surfaces, posing serious limitations in the fabrication of multilayered curved constructs. 4D bioprinting, combining 3D bioprinting with time-dependent stimuli-induced transformation, enables the fabrication of shape-changing constructs. Here, a 4D biofabrication method is reported for cartilage engineering based on the differential swelling of a smart multi-material system made from two hydrogel-based materials: hyaluronan and alginate. Two ink formulations are used: tyramine-functionalized hyaluronan (HAT, high-swelling) and alginate with HAT (AHAT, low-swelling). Both inks have similar elastic, shear-thinning, and printability behavior. The inks are 3D printed into a bilayered scaffold before triggering the shape-change by using liquid immersion as stimulus. In time (4D), the differential swelling between the two zones leads to the scaffold's self-bending. Different designs are made to tune the radius of curvature and shape. A bioprinted formulation of AHAT and human bone marrow cells demonstrates high cell viability. After 28 days in chondrogenic medium, the curvature is clearly present while cartilage-like matrix production is visible on histology. A proof-of-concept of the recently emerged technology of 4D bioprinting with a specific application for the design of curved structures potentially mimicking the curvature and multilayer cellular nature of native cartilage is demonstrated.
Keywords: 4D bioprinting; biofabrication; shape-change; smart bioinks; tissue engineering.
© 2022 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Cell-laden four-dimensional bioprinting using near-infrared-triggered shape-morphing alginate/polydopamine bioinks.Biofabrication. 2019 Sep 13;11(4):045019. doi: 10.1088/1758-5090/ab39c5. Biofabrication. 2019. PMID: 31394520
-
Chondroinductive Alginate-Based Hydrogels Having Graphene Oxide for 3D Printed Scaffold Fabrication.ACS Appl Mater Interfaces. 2020 Jan 29;12(4):4343-4357. doi: 10.1021/acsami.9b22062. Epub 2020 Jan 17. ACS Appl Mater Interfaces. 2020. PMID: 31909967
-
Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs.Acta Biomater. 2020 Apr 1;106:114-123. doi: 10.1016/j.actbio.2020.01.046. Epub 2020 Feb 3. Acta Biomater. 2020. PMID: 32027992
-
Alginate based hydrogel inks for 3D bioprinting of engineered orthopedic tissues.Carbohydr Polym. 2022 Nov 15;296:119964. doi: 10.1016/j.carbpol.2022.119964. Epub 2022 Aug 5. Carbohydr Polym. 2022. PMID: 36088004 Review.
-
4D printed shape-shifting biomaterials for tissue engineering and regenerative medicine applications.Biofabrication. 2024 Feb 9;16(2). doi: 10.1088/1758-5090/ad1e6f. Biofabrication. 2024. PMID: 38224616 Review.
Cited by
-
Organoids and tissue/organ chips.Stem Cell Res Ther. 2024 Aug 5;15(1):241. doi: 10.1186/s13287-024-03859-1. Stem Cell Res Ther. 2024. PMID: 39098898 Free PMC article. No abstract available.
-
Smart implants: 4D-printed shape-morphing scaffolds for medical implantation.Int J Bioprint. 2023 May 30;9(5):764. doi: 10.18063/ijb.764. eCollection 2023. Int J Bioprint. 2023. PMID: 37457930 Free PMC article. Review.
-
Transformative bioprinting: 4D printing and its role in the evolution of engineering and personalized medicine.Discov Nano. 2025 Jul 23;20(1):118. doi: 10.1186/s11671-025-04230-w. Discov Nano. 2025. PMID: 40699389 Free PMC article. Review.
-
Magnetically driven formation of 3D freestanding soft bioscaffolds.Sci Adv. 2024 Feb 2;10(5):eadl1549. doi: 10.1126/sciadv.adl1549. Epub 2024 Feb 2. Sci Adv. 2024. PMID: 38306430 Free PMC article.
-
Construction of organoids using bioprinting technology: a frontier exploration of cartilage repair.J Orthop Translat. 2025 Jul 16;54:37-50. doi: 10.1016/j.jot.2025.06.020. eCollection 2025 Sep. J Orthop Translat. 2025. PMID: 40703568 Free PMC article. Review.
References
-
- Costantini M., Testa S., Mozetic P., Barbetta A., Fuoco C., Fornetti E., Tamiro F., Bernardini S., Jaroszewicz J., Święszkowski W., Trombetta M., Castagnoli L., Seliktar D., Garstecki P., Cesareni G., Cannata S., Rainer A., Gargioli C., Biomaterials 2017, 131, 98. - PubMed
-
- Lee A., Hudson A. R., Shiwarski D. J., Tashman J. W., Hinton T. J., Yerneni S., Bliley J. M., Campbell P. G., Feinberg A. W., Science 2019, 365, 482. - PubMed
-
- Groll J., Boland T., Blunk T., Burdick J. A., Cho D.‐W., Dalton P. D., Derby B., Forgacs G., Li Q., Mironov V. A., Moroni L., Nakamura M., Shu W., Takeuchi S., Vozzi G., Woodfield T. B. F., Xu T., Yoo J. J., Malda J., Biofabrication 2016, 8, 013001. - PubMed
-
- Jiang T., Munguia‐Lopez J. G., Flores‐Torres S., Kort‐Mascort J., Kinsella J. M., Appl. Phys. Rev. 2019, 6, 011310.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

