Axin1: A novel scaffold protein joins the antiviral network of interferon
- PMID: 36308071
- PMCID: PMC9789182
- DOI: 10.1111/mmi.14995
Axin1: A novel scaffold protein joins the antiviral network of interferon
Abstract
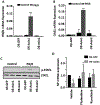
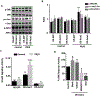
Acute respiratory infection by influenza virus is a persistent and pervasive public health problem. Antiviral innate immunity initiated by type I interferon (IFN) is the first responder to pathogen invasion and provides the first line of defense. We discovered that Axin1, a scaffold protein, was reduced during influenza virus infection. We also found that overexpression of Axin1 and the chemical stabilizer of Axin1, XAV939, reduced influenza virus replication in lung epithelial cells. This effect was also observed with respiratory syncytial virus and vesicular stomatitis virus. Axin1 boosted type I IFN response to influenza virus infection and activated JNK/c-Jun and Smad3 signaling. XAV939 protected mice from influenza virus infection. Thus, our studies provide new mechanistic insights into the regulation of the type I IFN response and present a new potential therapeutic of targeting Axin1 against influenza virus infection.
Keywords: Axin1; JNK/c-Jun; Smad3; influenza virus; interferon.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest:
The authors have no conflict of interest to declare
Figures

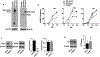
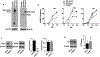

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

