A two-gene strategy increases iron and zinc concentrations in wheat flour, improving mineral bioaccessibility
- PMID: 36308454
- PMCID: PMC9806615
- DOI: 10.1093/plphys/kiac499
A two-gene strategy increases iron and zinc concentrations in wheat flour, improving mineral bioaccessibility
Abstract
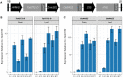
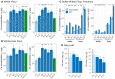
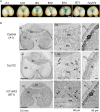
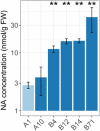
Dietary deficiencies of iron and zinc cause human malnutrition that can be mitigated by biofortified staple crops. Conventional breeding approaches to increase grain mineral concentrations in wheat (Triticum aestivum L.) have had only limited success, and our understanding of the genetic and physiological barriers to altering this trait is incomplete. Here we demonstrate that a transgenic approach combining endosperm-specific expression of the wheat VACUOLAR IRON TRANSPORTER gene TaVIT2-D with constitutive expression of the rice (Oryza sativa) NICOTIANAMINE SYNTHASE gene OsNAS2 significantly increases the total concentration of zinc and relocates iron to white-flour fractions. In two distinct bread wheat cultivars, we show that the so called VIT-NAS construct led to a two-fold increase in zinc in wholemeal flour, to ∼50 µg g-1. Total iron was not significantly increased, but redistribution within the grain resulted in a three-fold increase in iron in highly pure, roller-milled white flour, to ∼25 µg g-1. Interestingly, expression of OsNAS2 partially restored iron translocation to the aleurone, which is iron depleted in grain overexpressing TaVIT2 alone. A greater than three-fold increase in the level of the natural plant metal chelator nicotianamine in the grain of VIT-NAS lines corresponded with improved iron and zinc bioaccessibility in white flour. The growth of VIT-NAS plants in the greenhouse was indistinguishable from untransformed controls. Our results provide insights into mineral translocation and distribution in wheat grain and demonstrate that the individual and combined effects of the two transgenes can enhance the nutritional quality of wheat beyond what is possible by conventional breeding.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures

References
-
- Banakar R, Alvarez Fernández Á, Abadía J, Capell T, Christou P (2017) The expression of heterologous Fe (III) phytosiderophore transporter HvYS1 in rice increases Fe uptake, translocation and seed loading and excludes heavy metals by selective Fe transport. Plant Biotechnol J 15: 423–432 - PMC - PubMed
-
- Beasley JT, Bonneau JP, Moreno-Moyano LT, Callahan DL, Howell KS, Tako E, Taylor J, Glahn RP, Appels R, Johnson AAT (2021) Multi-year field evaluation of nicotianamine biofortified bread wheat. Plant J 109: 1168–1182 - PubMed
-
- Borg S (2012) Wheat ferritins: improving the iron content of the wheat grain. J Cereal Sci 56: 204–213
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

