Pubertal development underlies optimization of inhibitory control through specialization of ventrolateral prefrontal cortex
- PMID: 36308857
- PMCID: PMC9618767
- DOI: 10.1016/j.dcn.2022.101162
Pubertal development underlies optimization of inhibitory control through specialization of ventrolateral prefrontal cortex
Abstract
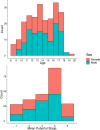
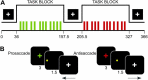
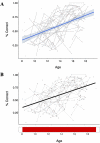
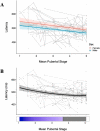
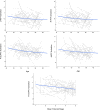
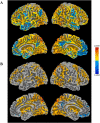
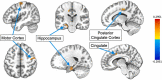
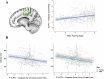
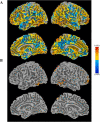
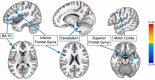
Inhibitory control improves into young adulthood after specialization of relevant brain systems during adolescence. However, the biological mechanisms supporting this unique transition are not well understood. Given that adolescence is defined by puberty, we examined relative contributions of chronological age and pubertal maturation to inhibitory control development. 105 8-19-year-olds completed 1-5 longitudinal visits (227 visits total) in which pubertal development was assessed via self-reported Tanner stage and inhibitory control was assessed with an in-scanner antisaccade task. As expected, percentage and latency of correct antisaccade responses improved with age and pubertal stage. When controlling for pubertal stage, chronological age was distinctly associated with correct response rate. In contrast, pubertal stage was uniquely associated with antisaccade latency even when controlling for age. Chronological age was associated with fMRI task activation in several regions including the right dorsolateral prefrontal cortex, while puberty was associated with right ventrolateral prefrontal cortex (VLPFC) activation. Furthermore, task-related connectivity between VLPFC and cingulate was associated with both pubertal stage and response latency. These results suggest that while age-related developmental processes may support maturation of brain systems underlying the ability to inhibit a response, puberty may play a larger role in the effectiveness of generating cognitive control responses.
Keywords: Adolescence; Antisaccade; Inhibitory control; PPI; Puberty; fMRI.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Neural systems underlying reward cue processing in early adolescence: The role of puberty and pubertal hormones.Psychoneuroendocrinology. 2019 Apr;102:281-291. doi: 10.1016/j.psyneuen.2018.12.016. Epub 2018 Dec 13. Psychoneuroendocrinology. 2019. PMID: 30639923 Free PMC article.
-
Age related prefrontal compensatory mechanisms for inhibitory control in the antisaccade task.Neuroimage. 2018 Jan 15;165:92-101. doi: 10.1016/j.neuroimage.2017.10.001. Epub 2017 Oct 5. Neuroimage. 2018. PMID: 28988829
-
Testosterone during Puberty Shifts Emotional Control from Pulvinar to Anterior Prefrontal Cortex.J Neurosci. 2016 Jun 8;36(23):6156-64. doi: 10.1523/JNEUROSCI.3874-15.2016. J Neurosci. 2016. PMID: 27277794 Free PMC article.
-
Pubertal onset as a critical transition for neural development and cognition.Brain Res. 2017 Jan 1;1654(Pt B):87-94. doi: 10.1016/j.brainres.2016.04.012. Epub 2016 Apr 6. Brain Res. 2017. PMID: 27060769 Free PMC article. Review.
-
The role of puberty in the developing adolescent brain.Hum Brain Mapp. 2010 Jun;31(6):926-33. doi: 10.1002/hbm.21052. Hum Brain Mapp. 2010. PMID: 20496383 Free PMC article. Review.
Cited by
-
Mental Disorders, Social Media Addiction, and Academic Performance in Romanian Undergraduate Nursing Students.J Clin Med. 2024 Jul 31;13(15):4475. doi: 10.3390/jcm13154475. J Clin Med. 2024. PMID: 39124742 Free PMC article.
-
Common and unique network basis for externally and internally driven flexibility in cognition: From a developmental perspective.Dev Cogn Neurosci. 2025 Apr;72:101528. doi: 10.1016/j.dcn.2025.101528. Epub 2025 Feb 6. Dev Cogn Neurosci. 2025. PMID: 39929102 Free PMC article.
-
The influence of relative pubertal maturity on executive function development in adolescent girls.Sci Rep. 2024 Nov 15;14(1):28140. doi: 10.1038/s41598-024-71768-7. Sci Rep. 2024. PMID: 39548095 Free PMC article.
-
Impact of stress on excitatory and inhibitory markers of adolescent cognitive critical period plasticity.Neurosci Biobehav Rev. 2023 Oct;153:105378. doi: 10.1016/j.neubiorev.2023.105378. Epub 2023 Aug 27. Neurosci Biobehav Rev. 2023. PMID: 37643681 Free PMC article. Review.
-
The Effect of Pubertal Status on Self-Regulation of Behavior and Executive Functions-A Systematic Review.Dev Psychobiol. 2025 Sep;67(5):e70069. doi: 10.1002/dev.70069. Dev Psychobiol. 2025. PMID: 40790997 Free PMC article. Review.
References
-
- Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. - DOI
-
- Barendse M.E.A., Byrne M.L., Flournoy J.C., McNeilly E.A., Guazzelli Williamson V., Barrett A.-M.Y., Chavez S.J., Shirtcliff E.A., Allen N.B., Pfeifer J.H. Multimethod assessment of pubertal timing and associations with internalizing psychopathology in early adolescent girls. J. Psychopathol. Clin. Sci. 2022;131:14–25. doi: 10.1037/abn0000721. - DOI - PMC - PubMed
-
- Bates D., Mächler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2014;67:1–48.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

