Timeliness of reporting of SARS-CoV-2 seroprevalence results and their utility for infectious disease surveillance
- PMID: 36308993
- PMCID: PMC9583624
- DOI: 10.1016/j.epidem.2022.100645
Timeliness of reporting of SARS-CoV-2 seroprevalence results and their utility for infectious disease surveillance
Abstract
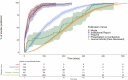
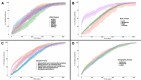
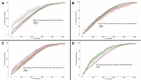
Seroprevalence studies have been used throughout the COVID-19 pandemic to monitor infection and immunity. These studies are often reported in peer-reviewed journals, but the academic writing and publishing process can delay reporting and thereby public health action. Seroprevalence estimates have been reported faster in preprints and media, but with concerns about data quality. We aimed to (i) describe the timeliness of SARS-CoV-2 serosurveillance reporting by publication venue and study characteristics and (ii) identify relationships between timeliness, data validity, and representativeness to guide recommendations for serosurveillance efforts. We included seroprevalence studies published between January 1, 2020 and December 31, 2021 from the ongoing SeroTracker living systematic review. For each study, we calculated timeliness as the time elapsed between the end of sampling and the first public report. We evaluated data validity based on serological test performance and correction for sampling error, and representativeness based on the use of a representative sample frame and adequate sample coverage. We examined how timeliness varied with study characteristics, representativeness, and data validity using univariate and multivariate Cox regression. We analyzed 1844 studies. Median time to publication was 154 days (IQR 64-255), varying by publication venue (journal articles: 212 days, preprints: 101 days, institutional reports: 18 days, and media: 12 days). Multivariate analysis confirmed the relationship between timeliness and publication venue and showed that general population studies were published faster than special population or health care worker studies; there was no relationship between timeliness and study geographic scope, geographic region, representativeness, or serological test performance. Seroprevalence studies in peer-reviewed articles and preprints are published slowly, highlighting the limitations of using the academic literature to report seroprevalence during a health crisis. More timely reporting of seroprevalence estimates can improve their usefulness for surveillance, enabling more effective responses during health emergencies.
Keywords: Bibliometrics; COVID-19; Infectious disease; Public health surveillance; Reporting; Seroprevalence.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Interest Rahul K. Arora was previously a Technical Consultant for the Bill and Melinda Gates Foundation Strategic Investment Fund, is a minority shareholder of Alethea Medical, and was a former Senior Policy Advisor at Health Canada. David Buckeridge consults for the Public Health Agency of Canada and has participated with Medicago. David Clifton consults for Oxford University Innovation, Biobeats, Sensyne Health, and Bristol Myers Squibb. Each of these relationships is entirely unrelated to the present work. No other authors have conflicts of interest to report.
Figures

References
-
- Bergeri I., Whelan M., Ware H., Subissi L., Nardone A., Lewis H.C., Li Z., Ma X., Valenciano M., Cheng B., Al Ariqi L., Rashidian A., Okeibunor J., Azim T., Wijesinghe P., Le L.V., Vaughan A., Pebody R., Vicari A., Yan T., Yanes-Lane M., Cao C., Cheng M.P., Papenburg J., Buckeridge D., Bobrovitz N., Arora R.K., van Kerkhove M.D. Global epidemiology of SARS-CoV-2 infection: a systematic review and meta-analysis of standardized population-based seroprevalence studies, Jan 2020-Oct 2021 (preprint) Epidemiology. 2021 doi: 10.1101/2021.12.14.21267791. (Unity Studies Collaborator Group) - DOI
-
- Bobrovitz N., Arora R.K., Cao C., Boucher E., Liu M., Rahim H., Donnici C., Ilincic N., Duarte N., Van Wyk J., Yan T., Penny L., Segal M., Chen J., Whelan M., Atmaja A., Rocco S., Joseph A., Clifton D.A., Williamson T., Yansouni C.P., Evans T.G., Chevrier J., Papenburg J., Cheng M.P. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS ONE. 2021:16. doi: 10.1101/2020.11.17.20233460. - DOI - PMC - PubMed
-
- Bobrovitz, N., Noël, K., Li, Z., Cao, C., Deveaux, G., Selemon, A., Clifton, D.A., Yanes-Lane, M., Yan, T., Arora, R.K., 2022. SeroTracker-RoB: an approach to automating reproducible risk of bias assessment of seroprevalence studies. https://doi.org/10.1101/2021.11.17.21266471. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

