The Toxoplasma effector GRA28 promotes parasite dissemination by inducing dendritic cell-like migratory properties in infected macrophages
- PMID: 36309013
- PMCID: PMC9710525
- DOI: 10.1016/j.chom.2022.10.001
The Toxoplasma effector GRA28 promotes parasite dissemination by inducing dendritic cell-like migratory properties in infected macrophages
Abstract
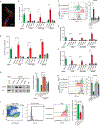
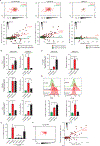
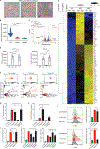
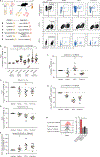
Upon pathogen detection, macrophages normally stay sessile in tissues while dendritic cells (DCs) migrate to secondary lymphoid tissues. The obligate intracellular protozoan Toxoplasma gondii exploits the trafficking of mononuclear phagocytes for dissemination via unclear mechanisms. We report that, upon T. gondii infection, macrophages initiate the expression of transcription factors normally attributed to DCs, upregulate CCR7 expression with a chemotactic response, and perform systemic migration when adoptively transferred into mice. We show that parasite effector GRA28, released by the MYR1 secretory pathway, cooperates with host chromatin remodelers in the host cell nucleus to drive the chemotactic migration of parasitized macrophages. During in vivo challenge studies, bone marrow-derived macrophages infected with wild-type T. gondii outcompeted those challenged with MYR1- or GRA28-deficient strains in migrating and reaching secondary organs. This work reveals how an intracellular parasite hijacks chemotaxis in phagocytes and highlights a remarkable migratory plasticity in differentiated cells of the mononuclear phagocyte system.
Keywords: apicomplexa; cell motility; chemokine receptor 7; chemotaxis; host-pathogen; immune evasion; intracellular parasitism; mononuclear phagocyte; protozoa.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Comment in
-
Macrophage to dendritic cell transitioning induced by Toxoplasma.Trends Parasitol. 2023 Jan;39(1):10-11. doi: 10.1016/j.pt.2022.11.007. Epub 2022 Dec 2. Trends Parasitol. 2023. PMID: 36470783
-
Hitching a ride in macrophages.Sci Signal. 2022 Dec 13;15(764):eadg1743. doi: 10.1126/scisignal.adg1743. Epub 2022 Dec 13. Sci Signal. 2022. PMID: 36512640
Similar articles
-
Hypermigration of macrophages through the concerted action of GRA effectors on NF-κB/p38 signaling and host chromatin accessibility potentiates Toxoplasma dissemination.mBio. 2024 Oct 16;15(10):e0214024. doi: 10.1128/mbio.02140-24. Epub 2024 Aug 29. mBio. 2024. PMID: 39207098 Free PMC article.
-
Hypermigration of macrophages through the concerted action of GRA effectors on NF-κB/p38 signaling and host chromatin accessibility potentiates Toxoplasma dissemination.bioRxiv [Preprint]. 2024 Feb 6:2024.02.06.579146. doi: 10.1101/2024.02.06.579146. bioRxiv. 2024. Update in: mBio. 2024 Oct 16;15(10):e0214024. doi: 10.1128/mbio.02140-24. PMID: 38370679 Free PMC article. Updated. Preprint.
-
The unicellular eukaryotic parasite Toxoplasma gondii hijacks the migration machinery of mononuclear phagocytes to promote its dissemination.Biol Cell. 2020 Sep;112(9):239-250. doi: 10.1111/boc.202000005. Epub 2020 May 19. Biol Cell. 2020. PMID: 32359185 Review.
-
Toxoplasma gondii GRA28 Is Required for Placenta-Specific Induction of the Regulatory Chemokine CCL22 in Human and Mouse.mBio. 2021 Dec 21;12(6):e0159121. doi: 10.1128/mBio.01591-21. Epub 2021 Nov 16. mBio. 2021. PMID: 34781732 Free PMC article.
-
Calling in the CaValry-Toxoplasma gondii Hijacks GABAergic Signaling and Voltage-Dependent Calcium Channel Signaling for Trojan horse-Mediated Dissemination.Front Cell Infect Microbiol. 2019 Mar 20;9:61. doi: 10.3389/fcimb.2019.00061. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30949456 Free PMC article. Review.
Cited by
-
Animal venoms: a novel source of anti-Toxoplasma gondii drug candidates.Front Pharmacol. 2023 May 3;14:1178070. doi: 10.3389/fphar.2023.1178070. eCollection 2023. Front Pharmacol. 2023. PMID: 37205912 Free PMC article. Review.
-
Seeing the unseen: illuminating Toxoplasma gondii's metabolic manipulation.mBio. 2024 Aug 14;15(8):e0121124. doi: 10.1128/mbio.01211-24. Epub 2024 Jul 12. mBio. 2024. PMID: 38995035 Free PMC article.
-
Dual single-cell and bulk RNA sequencing reveal transcriptional profiles underlying heterogenous host-parasite interactions in human peripheral blood mononuclear cells.Front Immunol. 2025 Jun 24;16:1582645. doi: 10.3389/fimmu.2025.1582645. eCollection 2025. Front Immunol. 2025. PMID: 40630957 Free PMC article.
-
Toxoplasma protein export and effector function.Nat Microbiol. 2024 Jan;9(1):17-28. doi: 10.1038/s41564-023-01563-z. Epub 2024 Jan 3. Nat Microbiol. 2024. PMID: 38172621 Review.
-
Fam96a is essential for the host control of Toxoplasma gondii infection by fine-tuning macrophage polarization via an iron-dependent mechanism.PLoS Negl Trop Dis. 2024 May 7;18(5):e0012163. doi: 10.1371/journal.pntd.0012163. eCollection 2024 May. PLoS Negl Trop Dis. 2024. PMID: 38713713 Free PMC article.

