Application of AOPs to assist regulatory assessment of chemical risks - Case studies, needs and recommendations
- PMID: 36309218
- PMCID: PMC9850416
- DOI: 10.1016/j.envres.2022.114650
Application of AOPs to assist regulatory assessment of chemical risks - Case studies, needs and recommendations
Abstract
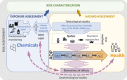
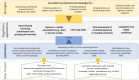
While human regulatory risk assessment (RA) still largely relies on animal studies, new approach methodologies (NAMs) based on in vitro, in silico or non-mammalian alternative models are increasingly used to evaluate chemical hazards. Moreover, human epidemiological studies with biomarkers of effect (BoE) also play an invaluable role in identifying health effects associated with chemical exposures. To move towards the next generation risk assessment (NGRA), it is therefore crucial to establish bridges between NAMs and standard approaches, and to establish processes for increasing mechanistically-based biological plausibility in human studies. The Adverse Outcome Pathway (AOP) framework constitutes an important tool to address these needs but, despite a significant increase in knowledge and awareness, the use of AOPs in chemical RA remains limited. The objective of this paper is to address issues related to using AOPs in a regulatory context from various perspectives as it was discussed in a workshop organized within the European Union partnerships HBM4EU and PARC in spring 2022. The paper presents examples where the AOP framework has been proven useful for the human RA process, particularly in hazard prioritization and characterization, in integrated approaches to testing and assessment (IATA), and in the identification and validation of BoE in epidemiological studies. Nevertheless, several limitations were identified that hinder the optimal usability and acceptance of AOPs by the regulatory community including the lack of quantitative information on response-response relationships and of efficient ways to map chemical data (exposure and toxicity) onto AOPs. The paper summarizes suggestions, ongoing initiatives and third-party tools that may help to overcome these obstacles and thus assure better implementation of AOPs in the NGRA.
Keywords: Adverse outcome pathways; Biomarkers of effect; Hazard assessment; Mechanistic toxicology; New approach methodologies; Regulatory risk assessment.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Andersen M., Anderson H., Bailar J.C., Boekelheide K., Brent R., Charnley G., Cheung V.G., Green S., Kelsey K.T., Kerkvliet N.I., Li A. a, McCray L., Meyer O., Patterson R.D., Pennie W., Scala R. a, Solomon G.M., Stephens M., Yager J., Zeise L., Daniel Krewski Daniel Acosta, Jr., Andersen Melvin, Anderson Henry, Bailar John C., III, Kim Boekelheide, Brent Robert, Charnley Gail, Vivian G.Cheung Sidney Green Jr. Kelsey Karl T., Kerkvliet Nancy I., Li Abby A., Lawrence McCray. Toxicity testing in the 21St century: a vision and a strategy. Natl. Acad. Sci. 2007;13:51–138. doi: 10.1080/10937404.2010.483176.TOXICITY. - DOI - PMC - PubMed
-
- Ankley G.T., Bennett R.S., Erickson R.J., Hoff D.J., Hornung M.W., Johnson R.D., Mount D.R., Nichols J.W., Russom C.L., Schmieder P.K., Serrrano J.A., Tietge J.E., Villeneuve D.L. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010;29:730–741. doi: 10.1002/etc.34. - DOI - PubMed
-
- Audouze K., Sarigiannis D., Alonso-Magdalena P., Brochot C., Casas M., Vrijheid M., Babin P.J., Karakitsios S., Coumoul X., Barouki R. Integrative strategy of testing systems for identification of endocrine disruptors inducing metabolic disorders—an introduction to the oberon project. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21082988. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

