In vivo ligamentogenesis in embroidered poly(lactic-co-ε-caprolactone) / polylactic acid scaffolds functionalized by fluorination and hexamethylene diisocyanate cross-linked collagen foams
- PMID: 36309635
- PMCID: PMC10006054
- DOI: 10.1007/s00418-022-02156-3
In vivo ligamentogenesis in embroidered poly(lactic-co-ε-caprolactone) / polylactic acid scaffolds functionalized by fluorination and hexamethylene diisocyanate cross-linked collagen foams
Abstract
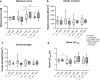
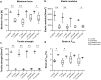
Although autografts represent the gold standard for anterior cruciate ligament (ACL) reconstruction, tissue-engineered ACLs provide a prospect to minimize donor site morbidity and limited graft availability. This study characterizes the ligamentogenesis in embroidered poly(L-lactide-co-ε-caprolactone) (P(LA-CL)) / polylactic acid (PLA) constructs using a dynamic nude mice xenograft model. (P(LA-CL))/PLA scaffolds remained either untreated (co) or were functionalized by gas fluorination (F), collagen foam cross-linked with hexamethylene diisocyanate (HMDI) (coll), or F combined with the foam (F + coll). Cell-free constructs or those seeded for 1 week with lapine ACL ligamentocytes were implanted into nude mice for 12 weeks. Following explantation, cell vitality and content, histo(patho)logy of scaffolds (including organs: liver, kidney, spleen), sulphated glycosaminoglycan (sGAG) contents and biomechanical properties were assessed.Scaffolds did not affect mice weight development and organs, indicating no organ toxicity. Moreover, scaffolds maintained their size and shape and reflected a high cell viability prior to and following implantation. Coll or F + coll scaffolds seeded with cells yielded superior macroscopic properties compared to the controls. Mild signs of inflammation (foreign-body giant cells and hyperemia) were limited to scaffolds without collagen. Microscopical score values and sGAG content did not differ significantly. Although remaining stable after explantation, elastic modulus, maximum force, tensile strength and strain at Fmax were significantly lower in explanted scaffolds compared to those before implantation, with no significant differences between scaffold subtypes, except for a higher maximum force in F + coll compared with F samples (in vivo). Scaffold functionalization with fluorinated collagen foam provides a promising approach for ACL tissue engineering. a Lapine anterior cruciate ligament (LACL): red arrow, posterior cruciate ligament: yellow arrow. Medial anterior meniscotibial ligament: black arrow. b Explant culture to isolate LACL fibroblasts. c Scaffold variants: co: controls; F: functionalization by gas-phase fluorination; coll: collagen foam cross-linked with hexamethylene diisocyanate (HMDI). c1-2 Embroidery pattern of the scaffolds. d Scaffolds were seeded with LACL fibroblasts using a dynamical culturing approach as depicted. e Scaffolds were implanted subnuchally into nude mice, fixed at the nuchal ligament and sacrospinal muscle tendons. f Two weeks after implantation. g Summary of analyses performed. Scale bars 1 cm (b, d), 0.5 cm (c). (sketches drawn by G.S.-T. using Krita 4.1.7 [Krita foundation, The Netherlands]).
Keywords: Anterior cruciate ligament; Dynamic nude mice xenograft model; Embroidered; Ligamentogenesis; Poly(L-lactide-co-ε-caprolactone) (P(LA-CL)); Polylactic acid (PLA) scaffolds; Tissue engineering.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
All authors declared that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Breier AC. Embroidery technology for hard-tissue scaffolds. Biomedical Textiles for Orthopaedic and Surgical Applications: Fundamentals, Applications and Tissue Engineering, Woodhead Publishing Series in Biomaterials; 2015. pp. 23–43.
-
- Cédric Laurent XL, De Isla N, Wang X, Rahouadj R. Defining a scaffold for ligament tissue engineering: What has been done, and what still needs to be done. J Cellular Immunotherapy. 2018;4:4–9. doi: 10.1016/j.jocit.2018.09.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

