Pyrotinib combined with trastuzumab and chemotherapy for the treatment of human epidermal growth factor receptor 2-positive metastatic breast cancer: a single-arm exploratory phase II trial
- PMID: 36309908
- PMCID: PMC9823079
- DOI: 10.1007/s10549-022-06770-6
Pyrotinib combined with trastuzumab and chemotherapy for the treatment of human epidermal growth factor receptor 2-positive metastatic breast cancer: a single-arm exploratory phase II trial
Abstract
Purpose: A substantial need for effective and safe treatment options is still unmet for patients with heavily pre-treated human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC). Herein, we assessed the efficacy and safety of pyrotinib plus trastuzumab and chemotherapy in patients with heavily treated HER2-positive MBC.
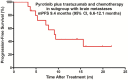
Methods: In this single-arm exploratory phase II trial, patients with HER2-positive MBC previously treated with trastuzumab plus lapatinib or pertuzumab, received pyrotinib plus trastuzumab and chemotherapy. The primary end point was progression-free survival (PFS) in the total population (TP). Secondary end points included PFS in the subgroup with brain metastases (Sub-BrM), confirmed objective response rate (ORR), clinical benefit rate (CBR), disease control rate (DCR), exploration of predictive factors of PFS, and safety.
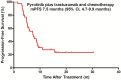
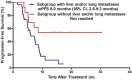
Results: Between November 1, 2018, and March 31, 2021, 40 patients were eligible for this study. The median PFS reached 7.5 months (95% confidence interval [CI] 4.7 to 9.9 months) and 9.4 months (95% CI 6.6 to 12.1 months) in the TP and Sub-BrM, respectively. ORR was 50.5% (20/40). CBR was 75.5% (30/40) and DCR reached 97.5% (39/40). Cox univariate and multivariate analyses demonstrated that liver or/and lung metastases was the significant adverse prognostic factor for PFS (p = 0.018; p = 0.026; respectively). The most frequent grade 3 or 4 treatment-related adverse events were diarrhea, neutropenia and leukopenia. No new safety signals were observed.
Conclusion: Pyrotinib plus trastuzumab and chemotherapy offered a promising option with manageable safety profile for heavily pre-treated HER2-positive MBC, especially for those without liver or/and lung metastases.
Keywords: Chemotherapy; Human epidermal growth factor receptor 2 (HER2); Metastatic breast cancer (MBC); Pyrotinib; Trastuzumab.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G, Untch M, Smith I, Gianni L, Baselga J, Al-Sakaff N, Lauer S, McFadden E, Leyland-Jones B, Bell R, Dowsett M, Jackisch C. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin adjuvant (HERA) trial. The Lancet. 2017;389:1195–1205. doi: 10.1016/s0140-6736(16)32616-2. - DOI - PMC - PubMed
-
- National Comprehensive Cancer Network (NCCN) (2021) Clinical practice guidelines in oncology: breast cancer. Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

