Jian-Pi-Yi-Shen decoction inhibits mitochondria-dependent granulosa cell apoptosis in a rat model of POF
- PMID: 36309912
- PMCID: PMC9648799
- DOI: 10.18632/aging.204320
Jian-Pi-Yi-Shen decoction inhibits mitochondria-dependent granulosa cell apoptosis in a rat model of POF
Erratum in
-
Correction for: Jian-Pi-Yi-Shen decoction inhibits mitochondria-dependent granulosa cell apoptosis in a rat model of POF.Aging (Albany NY). 2023 Mar 30;15(6):2358-2360. doi: 10.18632/aging.204637. Epub 2023 Mar 30. Aging (Albany NY). 2023. PMID: 36997328 Free PMC article. No abstract available.
Abstract
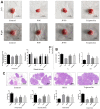
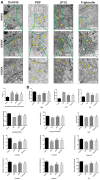
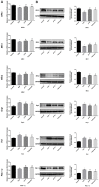
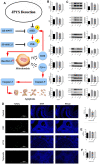
As a widely applied traditional Chinese medicine (TCM), Jian-Pi-Yi-Shen (JPYS) decoction maybe applied in curing premature ovarian failure (POF) besides chronic kidney disease (CKD). In vivo experiments, 40 female SD (8-week-old) rats were randomized into four groups, namely, control group (negative control), POF model group, JPYS treatment group, and triptorelin treatment group (positive control). JPYS group was treated with JPYS decoction (oral, 11 g/kg) for 60 days, and the triptorelin group was treated with triptorelin (injection, 1.5 mg/kg) for 10 days before the administration of cyclophosphamide (CTX) (50 mg/kg body weight) to establish POF model. We examined apoptosis, mitochondrial function, and target gene (ASK1/JNK pathway and mitochondrial fusion/fission) expression. In vitro experiments, the KGN human granulosa cell line was used. Cells were pretreated with CTX (20, 40, and 60 μg/mL) for 24 h, followed by JPYS-containing serum (2, 4, and 8 %) for 24 h. Thereafter, these cells were employed to assess apoptosis, mitochondrial function, and target gene levels of protein and mRNA. In vivo, JPYS alleviated injury and suppressed apoptosis in POF rats. In addition, JPYS improved ovarian function. JPYS inhibit apoptosis of granulosa cells through improving mitochondrial function by activating ASK1/JNK pathway. In vitro, JPYS inhibited KGN cell apoptosis through inhibited ASK1/JNK pathway and improved mitochondrial function. The effects of GS-49977 were similar to those of JPYS. During POF, mitochondrial dysfunction occurs in the ovary and leads to granulosa cell apoptosis. JPYS decoction improves mitochondrial function and alleviates apoptosis through ASK1/JNK pathway.
Keywords: Jian-Pi-Yi-Shen; apoptosis; granulosa cell; mitochondrial dysfunction; premature ovarian failure.
Conflict of interest statement
Figures



Similar articles
-
A Chinese herbal formula, Jian-Pi-Yi-Shen decoction, improves muscle atrophy via regulating mitochondrial quality control process in 5/6 nephrectomised rats.Sci Rep. 2017 Aug 23;7(1):9253. doi: 10.1038/s41598-017-10027-4. Sci Rep. 2017. Retraction in: Sci Rep. 2021 Jun 14;11(1):12831. doi: 10.1038/s41598-021-91869-x. PMID: 28835671 Free PMC article. Retracted.
-
Jian-Pi-Yi-Shen formula ameliorates renal fibrosis-induced anemia in rats with chronic kidney disease.J Ethnopharmacol. 2024 Dec 5;335:118607. doi: 10.1016/j.jep.2024.118607. Epub 2024 Jul 26. J Ethnopharmacol. 2024. PMID: 39069029
-
Distinct Responses of Gut Microbiota to Jian-Pi-Yi-Shen Decoction Are Associated With Improved Clinical Outcomes in 5/6 Nephrectomized Rats.Front Pharmacol. 2020 May 6;11:604. doi: 10.3389/fphar.2020.00604. eCollection 2020. Front Pharmacol. 2020. PMID: 32435197 Free PMC article.
-
Beneficial Effects of Traditional Chinese Medicine in the Treatment of Premature Ovarian Failure.Evid Based Complement Alternat Med. 2022 Nov 26;2022:5413504. doi: 10.1155/2022/5413504. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36471694 Free PMC article. Review.
-
Apoptotic mechanism of premature ovarian failure and rescue effect of Traditional Chinese Medicine: a review.J Tradit Chin Med. 2021 Jun;41(3):492-498. doi: 10.19852/j.cnki.jtcm.2021.03.017. J Tradit Chin Med. 2021. PMID: 34114409 Review.
Cited by
-
Transcription factor c-fos induces the development of premature ovarian insufficiency by regulating MALAT1/miR-22-3p/STAT1 network.J Ovarian Res. 2023 Jul 21;16(1):144. doi: 10.1186/s13048-023-01212-3. J Ovarian Res. 2023. PMID: 37480147 Free PMC article.
-
The risk factors, pathogenesis and treatment of premature ovarian insufficiency.J Ovarian Res. 2025 Jun 18;18(1):134. doi: 10.1186/s13048-025-01714-2. J Ovarian Res. 2025. PMID: 40533774 Free PMC article. Review.
-
Notch2 improves granulosa cell functions in premature ovarian failure by activating the Wnt2/β-catenin pathway.J Ovarian Res. 2025 Jul 30;18(1):169. doi: 10.1186/s13048-025-01745-9. J Ovarian Res. 2025. PMID: 40739650 Free PMC article.
-
The roles of MAPK signaling pathway in ovarian folliculogenesis.J Ovarian Res. 2025 Jul 14;18(1):152. doi: 10.1186/s13048-025-01737-9. J Ovarian Res. 2025. PMID: 40660328 Free PMC article. Review.
-
Mitochondria: the epigenetic regulators of ovarian aging and longevity.Front Endocrinol (Lausanne). 2024 Nov 13;15:1424826. doi: 10.3389/fendo.2024.1424826. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39605943 Free PMC article. Review.
References
-
- Siristatidis C, Dafopoulos K, Salamalekis G, Galazios G, Christoforidis N, Moustakarias T, Koutlaki N, Bouschanetzis C, Loutradis D, Drakakis P. Administration of low-molecular-weight heparin in patients with two or more unsuccessful IVF/ICSI cycles: a multicenter cohort study. Gynecol Endocrinol. 2018; 34:747–51. 10.1080/09513590.2018.1442426 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

