In Vitro Generation of Luminal Vasculature in Liver Organoids: From Basic Vascular Biology to Vascularized Hepatic Organoids
- PMID: 36310029
- PMCID: PMC9978835
- DOI: 10.15283/ijsc22154
In Vitro Generation of Luminal Vasculature in Liver Organoids: From Basic Vascular Biology to Vascularized Hepatic Organoids
Abstract
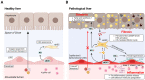
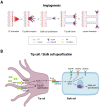
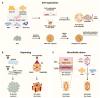
Liver organoids have gained much attention in recent years for their potential applications to liver disease modeling and pharmacologic drug screening. Liver organoids produced in vitro reflect some aspects of the in vivo physiological and pathological conditions of the liver. However, the generation of liver organoids with perfusable luminal vasculature remains a major challenge, hindering precise and effective modeling of liver diseases. Furthermore, vascularization is required for large organoids or assembloids to closely mimic the complexity of tissue architecture without cell death in the core region. A few studies have successfully generated liver organoids with endothelial cell networks, but most of these vascular networks produced luminal structures after being transplanted into tissues of host animals. Therefore, formation of luminal vasculature is an unmet need to overcome the limitation of liver organoids as an in vitro model investigating different acute and chronic liver diseases. Here, we provide an overview of the unique features of hepatic vasculature under pathophysiological conditions and summarize the biochemical and biophysical cues that drive vasculogenesis and angiogenesis in vitro. We also highlight recent progress in generating vascularized liver organoids in vitro and discuss potential strategies that may enable the generation of perfusable luminal vasculature in liver organoids.
Keywords: Blood vessel; Liver; Organoid; Stem cell; Vascularization.
Conflict of interest statement
The authors have no conflicting financial interest.
Figures
Similar articles
-
Generation of multilineage liver organoids with luminal vasculature and bile ducts from human pluripotent stem cells via modulation of Notch signaling.Stem Cell Res Ther. 2023 Feb 3;14(1):19. doi: 10.1186/s13287-023-03235-5. Stem Cell Res Ther. 2023. PMID: 36737811 Free PMC article.
-
Engineering the Vasculature of Stem-Cell-Derived Liver Organoids.Biomolecules. 2021 Jun 30;11(7):966. doi: 10.3390/biom11070966. Biomolecules. 2021. PMID: 34208902 Free PMC article. Review.
-
In vitro grafting of hepatic spheroids and organoids on a microfluidic vascular bed.Angiogenesis. 2022 Nov;25(4):455-470. doi: 10.1007/s10456-022-09842-9. Epub 2022 Jun 15. Angiogenesis. 2022. PMID: 35704148 Free PMC article.
-
Scalable production of tissue-like vascularized liver organoids from human PSCs.Exp Mol Med. 2023 Sep;55(9):2005-2024. doi: 10.1038/s12276-023-01074-1. Epub 2023 Sep 1. Exp Mol Med. 2023. PMID: 37653039 Free PMC article.
-
Central nervous system vascularization in human embryos and neural organoids.Cell Rep. 2024 Dec 24;43(12):115068. doi: 10.1016/j.celrep.2024.115068. Epub 2024 Dec 16. Cell Rep. 2024. PMID: 39693224 Free PMC article. Review.
Cited by
-
Construction of vascularized liver microtissues recapitulates angiocrine-mediated hepatocytes maturation and enhances therapeutic efficacy for acute liver failure.Bioact Mater. 2025 Apr 29;50:525-539. doi: 10.1016/j.bioactmat.2025.04.030. eCollection 2025 Aug. Bioact Mater. 2025. PMID: 40391105 Free PMC article.
-
Recent Advances in Three-Dimensional In Vitro Models for Studies of Liver Fibrosis.Tissue Eng Regen Med. 2025 Jul;22(5):593-609. doi: 10.1007/s13770-025-00719-8. Epub 2025 May 13. Tissue Eng Regen Med. 2025. PMID: 40358834 Review.
-
Guidelines for Manufacturing and Application of Organoids: Liver.Int J Stem Cells. 2024 May 30;17(2):120-129. doi: 10.15283/ijsc24044. Epub 2024 May 22. Int J Stem Cells. 2024. PMID: 38773747 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

