The Implication of Hinge 1 and Hinge 4 in Micro-Dystrophin Gene Therapy for Duchenne Muscular Dystrophy
- PMID: 36310439
- PMCID: PMC10210230
- DOI: 10.1089/hum.2022.180
The Implication of Hinge 1 and Hinge 4 in Micro-Dystrophin Gene Therapy for Duchenne Muscular Dystrophy
Abstract
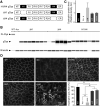
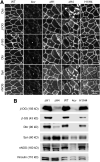
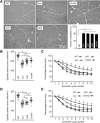
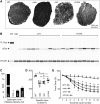
Duchenne muscular dystrophy (DMD) is a fatal muscle disease caused by dystrophin deficiency. Dystrophin consists of the amino terminus, central rod domain with 24 spectrin-like repeats and four hinges (H), cysteine-rich domain, and carboxyl terminus. Several highly abbreviated micro-dystrophins (μDys) are currently in clinical trials. They all carry H1 and H4. In this study, we investigated whether these two hinges are essential for μDy function in murine DMD models. Three otherwise identical μDys were engineered to contain H1 and/or H4 and were named H1/H4 (with both H1 and H4), ΔH1 (without H1), and ΔH4 (without H4). These constructs were packaged in adeno-associated virus serotype-9 and delivered to the tibialis anterior muscle of 3-month-old male mdx4cv mice (1E12 vector genome particles/muscle). Three months later, we detected equivalent μDys expression in total muscle lysate. However, only H1/H4 and ΔH1 showed correct sarcolemmal localization. ΔH4 mainly existed as sarcoplasmic aggregates. H1/H4 and ΔH1, but not ΔH4, fully restored the dystrophin-associated protein complex and significantly improved the specific muscle force. Eccentric contraction-induced force decline was best protected by H1/H4, followed by ΔH1, but not by ΔH4. Next, we compared H1/H4 and ΔH1 in 6-week-old male mdx mice by intravenous injection (1E13 vector genome particles/mouse). Four months postinjection, H1/H4 significantly outperformed ΔH1 in extensor digitorum longus muscle force measurements but two constructs yielded comparable electrocardiography improvements. We conclude that H4 is essential for μDys function and H1 facilitates force production. Our findings will help develop next-generation μDys gene therapy.
Keywords: Duchenne muscular dystrophy; dystrophin associated glycoprotein complex; hinges; mdx; micro-dystrophin; muscle function.
Conflict of interest statement
D.D. is a member of the Scientific Advisory Board for Solid Biosciences and equity holders of Solid Biosciences. D.D. is a member of the Scientific Advisory Board for Sardocor Corp. The Duan Laboratory received research support unrelated to this project from Solid Biosciences in the last 3 years. The Duan Laboratory has received research support unrelated to this project from Edgewise Therapeutics in the last 3 years. Other authors have no disclosure.
Figures

Similar articles
-
Development of Novel Micro-dystrophins with Enhanced Functionality.Mol Ther. 2019 Mar 6;27(3):623-635. doi: 10.1016/j.ymthe.2019.01.002. Epub 2019 Feb 1. Mol Ther. 2019. PMID: 30718090 Free PMC article.
-
Lifelong Outcomes of Systemic Adeno-Associated Virus Micro-Dystrophin Gene Therapy in a Murine Duchenne Muscular Dystrophy Model.Hum Gene Ther. 2023 May;34(9-10):449-458. doi: 10.1089/hum.2022.181. Epub 2023 May 2. Hum Gene Ther. 2023. PMID: 36515166 Free PMC article.
-
Long-term effect of human mini-dystrophin in transgenic mdx mice improves muscle physiological function.FASEB J. 2021 Jun;35(6):e21628. doi: 10.1096/fj.202100057RR. FASEB J. 2021. PMID: 33982338
-
[Gene therapy for muscular dystrophy].No To Hattatsu. 2004 Mar;36(2):117-23. No To Hattatsu. 2004. PMID: 15031985 Review. Japanese.
-
Systemic AAV Micro-dystrophin Gene Therapy for Duchenne Muscular Dystrophy.Mol Ther. 2018 Oct 3;26(10):2337-2356. doi: 10.1016/j.ymthe.2018.07.011. Epub 2018 Jul 17. Mol Ther. 2018. PMID: 30093306 Free PMC article. Review.
Cited by
-
Duchenne Muscular Dystrophy Gene Therapy in 2023: Status, Perspective, and Beyond.Hum Gene Ther. 2023 May;34(9-10):345-349. doi: 10.1089/hum.2023.29242.ddu. Hum Gene Ther. 2023. PMID: 37219994 Free PMC article.
-
In Silico Structural Prediction for the Generation of Novel Performant Midi-Dystrophins Based on Intein-Mediated Dual AAV Approach.Int J Mol Sci. 2024 Sep 27;25(19):10444. doi: 10.3390/ijms251910444. Int J Mol Sci. 2024. PMID: 39408775 Free PMC article.
-
Systemic delivery of full-length dystrophin in Duchenne muscular dystrophy mice.Nat Commun. 2024 Jul 21;15(1):6141. doi: 10.1038/s41467-024-50569-6. Nat Commun. 2024. PMID: 39034316 Free PMC article.
-
AAV microdystrophin gene replacement therapy for Duchenne muscular dystrophy: progress and prospects.Gene Ther. 2025 Aug 15. doi: 10.1038/s41434-025-00561-6. Online ahead of print. Gene Ther. 2025. PMID: 40817386 Review.
-
Duchenne muscular dystrophy treatment with lentiviral vector containing mini-dystrophin gene in vivo.MedComm (2020). 2024 Jan 6;5(1):e423. doi: 10.1002/mco2.423. eCollection 2024 Jan. MedComm (2020). 2024. PMID: 38188603 Free PMC article.
References
-
- Hoffman EP, Brown RH Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919–928. - PubMed
-
- Koenig M, Hoffman EP, Bertelson CJ, et al. . Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987;50:509–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

