Educational attainment, structural brain reserve and Alzheimer's disease: a Mendelian randomization analysis
- PMID: 36310536
- PMCID: PMC10151197
- DOI: 10.1093/brain/awac392
Educational attainment, structural brain reserve and Alzheimer's disease: a Mendelian randomization analysis
Abstract
Higher educational attainment is observationally associated with lower risk of Alzheimer's disease. However, the biological mechanisms underpinning this association remain unclear. The protective effect of education on Alzheimer's disease may be mediated via increased brain reserve. We used two-sample Mendelian randomization to explore putative causal relationships between educational attainment, structural brain reserve as proxied by MRI phenotypes and Alzheimer's disease. Summary statistics were obtained from genome-wide association studies of educational attainment (n = 1 131 881), late-onset Alzheimer's disease (35 274 cases, 59 163 controls) and 15 measures of grey or white matter macro- or micro-structure derived from structural or diffusion MRI (nmax = 33 211). We conducted univariable Mendelian randomization analyses to investigate bidirectional associations between (i) educational attainment and Alzheimer's disease; (ii) educational attainment and imaging-derived phenotypes; and (iii) imaging-derived phenotypes and Alzheimer's disease. Multivariable Mendelian randomization was used to assess whether brain structure phenotypes mediated the effect of education on Alzheimer's disease risk. Genetically proxied educational attainment was inversely associated with Alzheimer's disease (odds ratio per standard deviation increase in genetically predicted years of schooling = 0.70, 95% confidence interval 0.60, 0.80). There were positive associations between genetically predicted educational attainment and four cortical metrics (standard deviation units change in imaging phenotype per one standard deviation increase in genetically predicted years of schooling): surface area 0.30 (95% confidence interval 0.20, 0.40); volume 0.29 (95% confidence interval 0.20, 0.37); intrinsic curvature 0.18 (95% confidence interval 0.11, 0.25); local gyrification index 0.21 (95% confidence interval 0.11, 0.31)]; and inverse associations with cortical intracellular volume fraction [-0.09 (95% confidence interval -0.15, -0.03)] and white matter hyperintensities volume [-0.14 (95% confidence interval -0.23, -0.05)]. Genetically proxied levels of surface area, cortical volume and intrinsic curvature were positively associated with educational attainment [standard deviation units change in years of schooling per one standard deviation increase in respective genetically predicted imaging phenotype: 0.13 (95% confidence interval 0.10, 0.16); 0.15 (95% confidence interval 0.11, 0.19) and 0.12 (95% confidence interval 0.04, 0.19)]. We found no evidence of associations between genetically predicted imaging-derived phenotypes and Alzheimer's disease. The inverse association of genetically predicted educational attainment with Alzheimer's disease did not attenuate after adjusting for imaging-derived phenotypes in multivariable analyses. Our results provide support for a protective causal effect of educational attainment on Alzheimer's disease risk, as well as potential bidirectional causal relationships between education and brain macro- and micro-structure. However, we did not find evidence that these structural markers affect risk of Alzheimer's disease. The protective effect of education on Alzheimer's disease may be mediated via other measures of brain reserve not included in the present study, or by alternative mechanisms.
Keywords: Alzheimer’s disease; MRI; Mendelian randomization; brain reserve; educational attainment.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

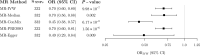


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

