Long noncoding RNA maternally expressed gene 3 improves trophoblast dysfunction and inflammation in preeclampsia through the Wnt/ β-Catenin/nod-like receptor pyrin domain-containing 3 axis
- PMID: 36310595
- PMCID: PMC9613960
- DOI: 10.3389/fmolb.2022.1022450
Long noncoding RNA maternally expressed gene 3 improves trophoblast dysfunction and inflammation in preeclampsia through the Wnt/ β-Catenin/nod-like receptor pyrin domain-containing 3 axis
Abstract
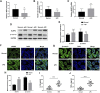
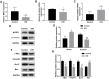
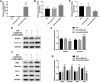
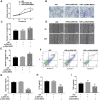
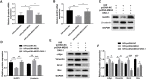
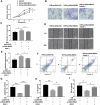
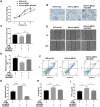
Inadequate trophoblastic infiltration and resulting placental hypoxia and inflammation comprise the core pathological basis of preeclampsia (PE). Maternally expressed gene 3 (MEG3) is known to be involved in the pathogenesis of preeclampsia by inhibiting the migration and invasion of trophoblasts and promoting their apoptosis. Nevertheless, the specific underlying downstream molecular mechanism of MEG3 is less well characterized. In this study, we detected lower expression levels of MEG3 and β-Catenin and higher expression of nod-like receptor pyrin domain-containing 3 (NLRP3) in placental tissues of pregnant women with severe preeclampsia (sPE) than in normal pregnancies. Elevated serum levels of IL-1β and TNF-α were also observed in the sPE group. Then, we established a hypoxia/reoxygenation (H/R) model to mimic preeclampsia. Similar results with sPE group were found in the H/R group compared with the control group. In addition, suppressive trophoblast proliferation, migration and invasion and increases in the apoptotic rate and inflammation were also detected in the H/R group. Notably, overexpressing MEG3 markedly improved trophoblast dysfunction and inflammation caused by H/R. However, the effects of MEG3 on trophoblasts, whether upregulated or downregulated, can be reversed by DKK-1 (Wnt/β-Catenin inhibitor) and MCC950 (NLRP3 inhibitor). The current study revealed that MEG3 regulates trophoblast function and inflammation through the Wnt/β-Catenin/NLRP3 axis and provided new insights into the pathogenesis of preeclampsia.
Keywords: NLRP3; Wnt/β-Catenin; inflammation; lncRNA MEG3; placenta; preeclampsia; trophoblast.
Copyright © 2022 Liang, Wang, Shi, Cui and Meng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Role and Molecular Mechanism of Long Nocoding RNA-MEG3 in the Pathogenesis of Preeclampsia.Reprod Sci. 2018 Dec;25(12):1619-1628. doi: 10.1177/1933719117749753. Epub 2018 Jan 23. Reprod Sci. 2018. PMID: 29361889
-
Downregulation of LncRNA-MEG3 promotes HTR8/SVneo cells apoptosis and attenuates its migration by repressing Notch1 signal in preeclampsia.Reproduction. 2020 Jul;160(1):21-29. doi: 10.1530/REP-19-0614. Reproduction. 2020. PMID: 32272451
-
[Expression and significance of SATB1 and wnt/β-catenin signaling molecule in the placenta of preeclampsia].Zhonghua Fu Chan Ke Za Zhi. 2015 Apr;50(4):283-90. Zhonghua Fu Chan Ke Za Zhi. 2015. PMID: 26080941 Chinese.
-
Wnt/β-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (Review).Mol Med Rep. 2017 Aug;16(2):1007-1013. doi: 10.3892/mmr.2017.6718. Epub 2017 Jun 7. Mol Med Rep. 2017. PMID: 29067442 Review.
-
Dysregulation of LncRNAs in Placenta and Pathogenesis of Preeclampsia.Curr Drug Targets. 2017;18(10):1165-1170. doi: 10.2174/1389450118666170404160000. Curr Drug Targets. 2017. PMID: 28382860 Review.
Cited by
-
Identification and preliminary validation of biomarkers associated with mitochondrial and programmed cell death in pre-eclampsia.Front Immunol. 2025 Jan 23;15:1453633. doi: 10.3389/fimmu.2024.1453633. eCollection 2024. Front Immunol. 2025. PMID: 39916955 Free PMC article.
-
A Narrative Review on the Pathophysiology of Preeclampsia.Int J Mol Sci. 2024 Jul 10;25(14):7569. doi: 10.3390/ijms25147569. Int J Mol Sci. 2024. PMID: 39062815 Free PMC article. Review.
-
Spatial multiomic landscape of the human placenta at molecular resolution.Nat Med. 2024 Dec;30(12):3495-3508. doi: 10.1038/s41591-024-03073-9. Epub 2024 Nov 20. Nat Med. 2024. PMID: 39567716
References
LinkOut - more resources
Full Text Sources

