Identification of new components of the basal pole of Toxoplasma gondii provides novel insights into its molecular organization and functions
- PMID: 36310866
- PMCID: PMC9613666
- DOI: 10.3389/fcimb.2022.1010038
Identification of new components of the basal pole of Toxoplasma gondii provides novel insights into its molecular organization and functions
Abstract
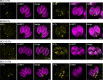
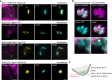
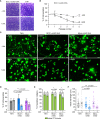
The Toxoplasma gondii tachyzoite is a singled-cell obligate intracellular parasite responsible for the acute phase of toxoplasmosis. This polarized cell exhibits an apical complex, a hallmark of the phylum Apicomplexa, essential for motility, invasion, and egress from the host cell. Located on the opposite end of the cell is the basal complex, an elaborated cytoskeletal structure that also plays critical roles in the lytic cycle of the parasite, being involved in motility, cell division, constriction and cytokinesis, as well as intravacuolar cell-cell communication. Nevertheless, only a few proteins of this structure have been described and functionally assessed. In this study, we used spatial proteomics to identify new basal complex components (BCC), and in situ imaging, including ultrastructure expansion microscopy, to position them. We thus confirmed the localization of nine BCCs out of the 12 selected candidates and assigned them to different sub-compartments of the basal complex, including two new domains located above the basal ring and below the posterior cup. Their functional investigation revealed that none of these BCCs are essential for parasite growth in vitro. However, one BCC is critical for constricting of the basal complex, likely through direct interaction with the class VI myosin heavy chain J (MyoJ), and for gliding motility. Four other BCCs, including a phosphatase and a guanylate-binding protein, are involved in the formation and/or maintenance of the intravacuolar parasite connection, which is required for the rosette organization and synchronicity of cell division.
Keywords: Apicomplexa; Toxoplasma gondii; basal complex; cell-cell communication; constriction; cytoskeleton; expansion microscopy; myosin heavy chain (MHC).
Copyright © 2022 Roumégous, Abou Hammoud, Fuster, Dupuy, Blancard, Salin, Robinson, Renesto, Tardieux and Frénal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Anderson-White B. R., Ivey F. D., Cheng K., Szatanek T., Lorestani A., Beckers C. J., et al. . (2011). A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii . Cell Microbiol. 13, 18–31. doi: 10.1111/j.1462-5822.2010.01514.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

