Anti-fibrillization effects of sulfonamide derivatives on α-synuclein and hyperphosphorylated tau isoform 1N4R
- PMID: 36310922
- PMCID: PMC9614895
- DOI: 10.1016/j.molstruc.2022.133574
Anti-fibrillization effects of sulfonamide derivatives on α-synuclein and hyperphosphorylated tau isoform 1N4R
Abstract
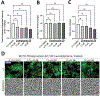
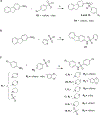
In contrast to Aβ plaques, the spatiotemporal distribution of neurofibrillary tangles of hyperphosphorylated tau (p-tau) predicts cognitive impairment in Alzheimer's disease (AD), underscoring the key pathological role of p-tau and the utmost need to develop AD therapeutics centering upon the control of p-tau aggregation and cytotoxicity. Our drug discovery program is focused on compounds that prevent the aggregation and cytotoxicity of p-tau moieties of the tau isoform 1N4R due to its prevalence (1 N) and long-distance trans-synaptic propagation (4R). We prepared and tested twenty-four newly synthesized small molecules representing the urea (1, 2, 3), sulfonylurea (4), and sulfonamide (5-24) series and evaluated their anti-aggregation effects with biophysical methods (thioflavin T and S fluorescence assays, transmission electron microscopy) and intracellular inclusion cell-based assays. Pre-evaluation was performed on alpha-synuclein (α-syn) to identify molecules to be challenged with p-tau. The sulfonamide derivatives 18 and 20 exhibited an anti-fribrillization activity on α-syn and p-tau. Sulfonamide compounds 18 and 20 reduced inclusion formation in M17D neuroblastoma cells that express inclusion-prone αSynuclein3K::YFP. This project advances new concepts in targeting prone-to-aggregate proteins such as α-syn and p-tau, and provides a molecular scaffold for further optimization and pre-clinical studies focused on AD drug development.
Keywords: Alpha-synuclein; Alzheimer’s disease; Fibrils; Hyperphosphorylated tau; Isoform 1N4R; Neuroblastoma cells; Sulfonamides.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interest.
Figures
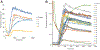





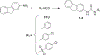

References
-
- Arriagada PV, Marzloff K, Hyman BT, Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease, Neurology 42 (9) (1992) 1681–1688. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

