Substance accumulation of a wetland plant, Leersia japonica, during senescence in the Yihe and Shuhe River Basin, North China
- PMID: 36311123
- PMCID: PMC9608780
- DOI: 10.3389/fpls.2022.996587
Substance accumulation of a wetland plant, Leersia japonica, during senescence in the Yihe and Shuhe River Basin, North China
Abstract
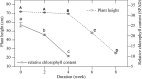
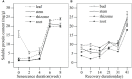
Leersia japonica is a perennial Gramineae grass that is dominant in shallow wetlands of the Yihe and Shuhe River Basin, North China. Previous studies have shown that L. japonica recovers early (March), tillers strongly, and has an excellent ability to purify sewage in spring. This early revival might play a vital role in water purification function; however, whether the plant benefits from the physiological activities during senescence remains unclear. Therefore, in this study, an experiment was conducted during the winter of 2016 and in the following spring. Morphology (height, biomass, root morphology), physiology (root vitality, malondialdehyde [MDA], superoxide dismutase [SOD]), substance contents (soluble sugar, soluble protein) and substance transportation (activity of enzymes for transportation and energy supply) were determined during weeks 0, 2, 4, 6, and 8 of the senescence stage (October 11, 2016); as well as substance contents and bud increments during days 0,7, 14, 21, 31 and 41 of the revival period (February 22, 2017). The results revealed that (1) the root biomass of L. japonica increased significantly during senescence, even after the leaves withered. (2) The root diameter of L. japonica decreased significantly, while root weight per volume and root superficial area per volume increased significantly during senescence. The root vitality was relatively stable in winter, especially for root absorption area per volume. (3) No significant difference was observed in membrane stability of stems, rhizomes and roots of L. japonica in winter, with the MDA content remaining stable and SOD activity increasing significantly during senescence. (4) The soluble sugar content of all tissues of L. japonica increased sharply during senescence; while it decreased significantly in spring, especially for buds. (5) The enzymes for substance metabolism responded differently, with activities of H+-ATPase and pyruvate decarboxylase (PDC) decreasing, and alcohol dehydrogenase (ADH) increasing. Therefore, L. japonica has active morphological adaptation of roots, physiological regulation, and massive substance accumulation during senescence stage. The special life-history trait ensures L. japonica survival in winter and revival in early spring, which makes it being an excellent plant for purifying sewage in spring.
Keywords: Leersia japonica; alcohol dehydrogenase; membrane stability; root vitality; substance transportation.
Copyright © 2022 Yang, Wang, Lei, Li and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Bao Y. Z., Xing J. P., Liang Y., Ren Z. P., Fu L. F., Yu J., et al. . (2022). Analysis of overwintering indexes ofwinter wheat in alpine regions and establishment of a cold resistance model. Field Crops Res. 275, 108347. doi: 10.1016/j.fcr.2021.108347 - DOI
-
- Brown J. M., Yu X., Holloway M. C. P., Dacosta M., Millammewis S. (2020). Differences in proteome response to cold acclimation in Zoysia japonica cultivars with different levels of freeze tolerance. Crop Sci. 60, 2744–2756. doi: 10.1002/csc2.20225 - DOI
-
- Che S. J., Zhao S. L. (2004). Forecast of beginning of flowering period of winter jasmine (Jasminum nudiflorum l.). Agric. Meteorol. 25, 70–73. doi: 10.1300/J064v24n01_09 - DOI
LinkOut - more resources
Full Text Sources

