A review of cardiac troponin I detection by surface enhanced Raman spectroscopy: Under the spotlight of point-of-care testing
- PMID: 36311415
- PMCID: PMC9608872
- DOI: 10.3389/fchem.2022.1017305
A review of cardiac troponin I detection by surface enhanced Raman spectroscopy: Under the spotlight of point-of-care testing
Abstract
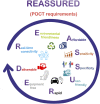
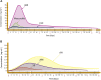
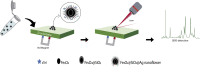
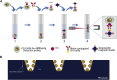
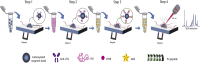
Cardiac troponin I (cTnI) is a biomarker widely related to acute myocardial infarction (AMI), one of the leading causes of death around the world. Point-of-care testing (POCT) of cTnI not only demands a short turnaround time for its detection but the highest accuracy levels to set expeditious and adequate clinical decisions. The analytical technique Surface-enhanced Raman spectroscopy (SERS) possesses several properties that tailor to the POCT format, such as its flexibility to couple with rapid assay platforms like microfluidics and paper-based immunoassays. Here, we analyze the strategies used for the detection of cTnI by SERS considering POCT requirements. From the detection ranges reported in the reviewed literature, we suggest the diseases other than AMI that could be diagnosed with this technique. For this, a section with information about cardiac and non-cardiac diseases with cTnI release, including their release kinetics or cut-off values are presented. Likewise, POCT features, the use of SERS as a POCT technique, and the biochemistry of cTnI are discussed. The information provided in this review allowed the identification of strengths and lacks of the available SERS-based point-of-care tests for cTnI and the disclosing of requirements for future assays design.
Keywords: SERS-based immunoassays; acute myocardial infarction (AMI); cardiac troponin I (cTnI); point-of-care testing; surface-enhanced Raman spectroscopy (SERS).
Copyright © 2022 Saviñon-Flores, Saviñon-Flores, Trejo, Méndez, Ţălu, González-Fuentes and Méndez-Albores.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

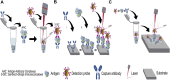





References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

