Targeted conditional collagen XII deletion alters tendon function
- PMID: 36311462
- PMCID: PMC9597098
- DOI: 10.1016/j.mbplus.2022.100123
Targeted conditional collagen XII deletion alters tendon function
Abstract
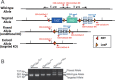
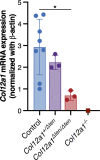
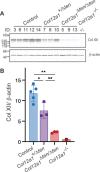
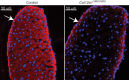
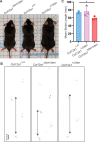
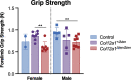
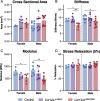
Collagen XII is a fibril-associated collagen with interrupted triple helices (FACIT). This non-fibrillar collagen is a homotrimer composed of three α1(XII) chains assembled into a collagenous molecule with a C terminal collagenous domain and a large N terminal non-collagenous domain. During tendon development and growth, collagen XII is broadly expressed throughout the extracellular matrix and enriched pericellularly around tenocytes. Tendons in a global Col12a1 -/- knockout model demonstrated disrupted fibril and fiber structure and disordered tenocyte organization, highlighting the critical regulatory roles of collagen XII in determining tendon structure and function. However, muscle and bone also are affected in the collagen XII knockout model. Therefore, secondary effects on tendon due to involvement of bone and muscle may occur in the global knockout. The global knockout does not allow the definition of intrinsic mechanisms involving collagen XII in tendon versus extrinsic roles involving muscle and bone. To address this limitation, we created and characterized a conditional Col12a1-null mouse model to permit the spatial and temporal manipulation of Col12a1 expression. Collagen XII knockout was targeted to tendons by breeding conditional Col12a1 flox/flox mice with Scleraxis-Cre (Scx-Cre) mice to yield a tendon-specific Col12a1-null mouse line, Col12a1 Δten/Δten . Both mRNA and protein expression in Col12a1 Δten/Δten mice decreased to near baseline levels in flexor digitorum longus tendons (FDL). Collagen XII immuno-localization revealed an absence of reactivity in the tendon proper, but there was reactivity in the cells of the surrounding peritenon. This supports a targeted knockout in tenocytes while peritenon cells from a non-tendon lineage were not targeted and retained collagen XII expression. The tendon-targeted, Col12a1 Δten/Δten mice had significantly reduced forelimb grip strength, altered gait and a significant decrease in biomechanical properties. While the observed decrease in tendon modulus suggests that differences in tendon material properties in the absence of Col12a1 expression underlie the functional deficiencies. Together, these findings suggest an intrinsic role for collagen XII critical for development of a functional tendon.
Keywords: Biomechanics; Col12a1; Collagen XII; Conditional mouse model; Tendon; Transgenic.
© 2022 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Collagen XII mediated cellular and extracellular mechanisms in development, regeneration, and disease.Front Cell Dev Biol. 2023 Mar 2;11:1129000. doi: 10.3389/fcell.2023.1129000. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36936682 Free PMC article. Review.
-
Collagen XI regulates the acquisition of collagen fibril structure, organization and functional properties in tendon.Matrix Biol. 2020 Dec;94:77-94. doi: 10.1016/j.matbio.2020.09.001. Epub 2020 Sep 17. Matrix Biol. 2020. PMID: 32950601 Free PMC article.
-
Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function.Matrix Biol. 2021 Jan;95:52-67. doi: 10.1016/j.matbio.2020.10.004. Epub 2020 Oct 20. Matrix Biol. 2021. PMID: 33096204 Free PMC article.
-
Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers-Danlos syndrome joint phenotype.Am J Pathol. 2015 May;185(5):1436-47. doi: 10.1016/j.ajpath.2015.01.031. Epub 2015 Mar 20. Am J Pathol. 2015. PMID: 25797646 Free PMC article.
-
Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils.Int J Biochem Cell Biol. 2014 Aug;53:51-4. doi: 10.1016/j.biocel.2014.04.020. Epub 2014 May 4. Int J Biochem Cell Biol. 2014. PMID: 24801612 Free PMC article. Review.
Cited by
-
The coordinated activities of collagen VI and XII in maintenance of tissue structure, function and repair: evidence for a physical interaction.Front Mol Biosci. 2024 Mar 28;11:1376091. doi: 10.3389/fmolb.2024.1376091. eCollection 2024. Front Mol Biosci. 2024. PMID: 38606288 Free PMC article.
-
Collagen XII mediated cellular and extracellular mechanisms in development, regeneration, and disease.Front Cell Dev Biol. 2023 Mar 2;11:1129000. doi: 10.3389/fcell.2023.1129000. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36936682 Free PMC article. Review.
-
Characterization of TGFβ1-induced tendon-like structure in the scaffold-free three-dimensional tendon cell culture system.Sci Rep. 2024 Apr 25;14(1):9495. doi: 10.1038/s41598-024-60221-4. Sci Rep. 2024. PMID: 38664570 Free PMC article.
-
Extracellular matrix protein composition dynamically changes during murine forelimb development.iScience. 2024 Jan 9;27(2):108838. doi: 10.1016/j.isci.2024.108838. eCollection 2024 Feb 16. iScience. 2024. PMID: 38303699 Free PMC article.
References
-
- Zhang G., Young B.B., Ezura Y., Favata M., Soslowsky L.J., Chakravarti S., Birk D.E. Development of tendon structure and function: regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal Interact. 2005;5(1):5–21. - PubMed
-
- Graham H.K., Holmes D.F., Watson R.B., Kadler K.E. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J. Mol. Biol. 2000;295(4):891–902. - PubMed
-
- Canty E.G., Kadler K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005;118(Pt 7):1341–1353. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

