Immunomagnetic separation of Toxoplasma gondii and Hammondia spp. tissue cysts generated in cell culture
- PMID: 36311681
- PMCID: PMC9606798
- DOI: 10.3389/fvets.2022.1033380
Immunomagnetic separation of Toxoplasma gondii and Hammondia spp. tissue cysts generated in cell culture
Abstract
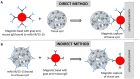
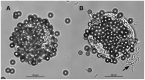
Toxoplasma gondii is commonly transmitted among animals and humans by ingestion of infected animal tissues or by consumption of food and water contaminated with environmentally-resistant oocysts excreted by cats. Tissue cysts and oocysts have different walls, whose structures and compositions are poorly known. Herein, we describe an immunomagnetic separation (IMS) method that was successfully used for purification of T. gondii tissue cysts generated in cell culture. We used an IgG monoclonal antibody (mAb) that reacts against antigens in tissue cyst walls. Many in vitro produced cysts were obtained by this IMS; >2,000 T. gondii cysts were isolated from a single culture flask of 25 cm2. Tissue cysts from two Hammondia spp., H. hammondi, and H. heydorni, produced in cell culture were also separated using this method. As a reference, purification of tissue cysts by Percoll gradients was used. Percoll was able to separate T. gondii tissue cysts produced in mice but was not suitable for purifying T. gondii tissue cysts produced in vitro. The IMS described here should favor proteomic studies involving tissue cysts of T. gondii.
Keywords: Hammondia hammondi; Hammondia heydorni; Toxoplasma gondii; immunomagnetic; monoclonal antibody; tissue cyst wall.
Copyright © 2022 Rezende-Gondim, da Silva, Dubey, Schares and Gondim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

