DNA-PKcs restricts Zika virus spreading and is required for effective antiviral response
- PMID: 36311766
- PMCID: PMC9606669
- DOI: 10.3389/fimmu.2022.1042463
DNA-PKcs restricts Zika virus spreading and is required for effective antiviral response
Abstract
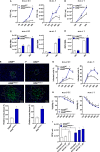
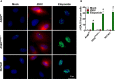
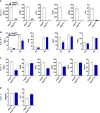
Zika virus (ZIKV) is a single-strand RNA mosquito-borne flavivirus with significant public health impact. ZIKV infection induces double-strand DNA breaks (DSBs) in human neural progenitor cells that may contribute to severe neuronal manifestations in newborns. The DNA-PK complex plays a critical role in repairing DSBs and in the innate immune response to infection. It is unknown, however, whether DNA-PK regulates ZIKV infection. Here we investigated the role of DNA-PKcs, the catalytic subunit of DNA-PK, during ZIKV infection. We demonstrate that DNA-PKcs restricts the spread of ZIKV infection in human epithelial cells. Increased ZIKV replication and spread in DNA-PKcs deficient cells is related to a notable decrease in transcription of type I and III interferons as well as IFIT1, IFIT2, and IL6. This was shown to be independent of IRF1, IRF3, or p65, canonical transcription factors necessary for activation of both type I and III interferon promoters. The mechanism of DNA-PKcs to restrict ZIKV infection is independent of DSB. Thus, these data suggest a non-canonical role for DNA-PK during Zika virus infection, acting downstream of IFNs transcription factors for an efficient antiviral immune response.
Keywords: DNA-PKcs; Zika virus; double-strand DNA breaks; infection; interferon.
Copyright © 2022 Patricio, Dias, Granella, Trigg, Teague, Bittencourt, Báfica, Zanotto-Filho, Ferguson and Mansur.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

