Impact of One-Week Administration of Dihydrotestosterone in Rat Anterior Pituitary Gland
- PMID: 36311909
- PMCID: PMC9616675
- DOI: 10.1155/2022/9525227
Impact of One-Week Administration of Dihydrotestosterone in Rat Anterior Pituitary Gland
Abstract
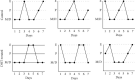
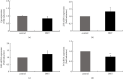
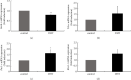
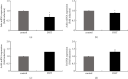
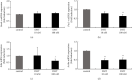
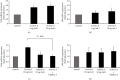
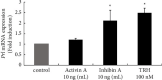
Hyperandrogenism causes dysfunction of the hypothalamic-pituitary-gonadal (HPG) axis in reproductive women. In this study, we examined the effects of dihydrotestosterone (DHT) on characteristic changes in rat anterior pituitary gland samples. DHT was administered to ovary-intact 6-week postnatal female rats for 7 days, after which the anterior pituitary glands were examined and compared with those in control rats. Estrous cyclicity was not drastically disrupted by DHT treatment. Common gonadotropin α subunit (Cga), luteinizing hormone β subunit (Lhb), and follicle-stimulating hormone (FSH) β subunit (Fshb) gene expression levels were not modulated by DHT treatment, while prolactin (Prl) gene expression was significantly repressed by DHT. Gonadotropin-releasing hormone (GnRH) receptor (Gnrh-r) gene expression was significantly inhibited by DHT, whereas pituitary adenylate cyclase-activating polypeptide (PACAP) receptor (Pca1-r) gene expression was increased by DHT. Gene expression levels of the receptors encoded by thyrotropin-releasing hormone (Trh-r) and kisspeptin (Kiss1-r) genes were unchanged. Expression of inhibin α subunit (Inha) and activin βA subunits (Actba) within the pituitary was inhibited by DHT treatment, while activin B subunit (Actbb) and follistatin (Fst) gene expression was unchanged by DHT. In mouse pituitary gonadotroph LβT2 cells, DHT did not modulate the gene expression of Gnrh-r, but it inhibited the expression of Inha and Actba subunits within the LβT2 cells. In rat prolactin-producing GH3 cells, DHT did not modulate prolactin gene expression, but it increased Pac1-r gene expression. The present observations suggest that DHT directly or indirectly affects the anterior pituitary gland and induces characteristic changes in hormone-producing cells.
Copyright © 2022 Haruhiko Kanasaki et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
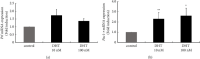
References
-
- Matheson E., Bain J. Hirsutism in women. American Family Physician . 2019;100(3):168–175. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

