Effect of N-Methyl-pyrrolidone (NMP) on the Equilibrium Solubility of Meloxicam in Aqueous Media: Correlation, Dissolution Thermodynamics, and Preferential Solvation
- PMID: 36312332
- PMCID: PMC9609070
- DOI: 10.1021/acsomega.2c05189
Effect of N-Methyl-pyrrolidone (NMP) on the Equilibrium Solubility of Meloxicam in Aqueous Media: Correlation, Dissolution Thermodynamics, and Preferential Solvation
Abstract
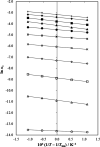
Meloxicam is an analgesic and anti-inflammatory drug widely prescribed in current therapeutics that exhibits very low solubility in water. Thus, this physicochemical property has been studied in N-methyl-pyrrolidone (NMP)-aqueous mixtures at several temperatures to expand the solubility database about pharmaceutical compounds in aqueous-mixed solvents. The flask-shake method and UV-vis spectrophotometry were used for meloxicam solubility determination as a function of temperature and mixture composition. Several cosolvency models, including the Jouyban-Acree model, were challenged for equilibrium solubility correlation and/or prediction. The van't Hoff and Gibbs equations were employed here to calculate the apparent standard thermodynamic quantities for the dissolution and mixing processes of this drug in these aqueous mixtures. Inverse Kirkwood-Buff integrals were employed to calculate the preferential solvation parameters of meloxicam by NMP in all mixtures. Meloxicam equilibrium solubility increased with increasing temperature, and maximal solubilities were observed in neat NMP at all temperatures. The mole fraction solubility of meloxicam increased from 1.137 × 10-6 in neat water to 3.639 × 10-3 in neat NMP at 298.15 K. The Jouyban-Acree model correlated the meloxicam solubility in these mixtures very well. Dissolution processes were endothermic and entropy-driven in all cases, except in neat water, where nonenthalpy- nor entropy-driven was observed. Apparent Gibbs energies of dissolution varied from 34.35 kJ·mol-1 in pure water to 7.99 kJ·mol-1 in pure NMP at a mean harmonic temperature of 303.0 K. A nonlinear enthalpy-entropy relationship was observed in the plot of dissolution enthalpy vs dissolution Gibbs energy. Meloxicam is preferentially hydrated in water-rich mixtures but preferentially solvated by NMP in the composition interval of 0.16 < x 1 < 1.00.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Budavari S.; O’Neil M. J.; Smith A.; Heckelman P. E.; Obenchain J. R. Jr; Gallipeau J. A. R.. et al.The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck & Co., Inc.: Whitehouse Station (NJ), 2001.
-
- Sweetman S. C.Martindale: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, 2009.
LinkOut - more resources
Full Text Sources
