Access to Diverse Seleno-spirocyclohexadienones via Ag(II)-Catalyzed Selenylative ipso-Annulation with Se and Boronic Acids
- PMID: 36312410
- PMCID: PMC9608386
- DOI: 10.1021/acsomega.2c05394
Access to Diverse Seleno-spirocyclohexadienones via Ag(II)-Catalyzed Selenylative ipso-Annulation with Se and Boronic Acids
Abstract
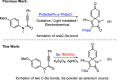
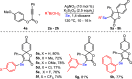
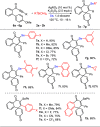
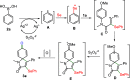
An efficient and straightforward synthesis of diversified seleno-azaspiro-2,5-cyclohexadienones from N-(4-methoxy aryl)propiolamides using elemental selenium and boronic acids has been demonstrated. The reaction proceeds through silver-catalyzed oxidative dearomatization in the presence of potassium persulfate (K2S2O8) as the oxidant. Further, this approach was extended to N-(4-methoxy aryl)propiolates and biaryl ynones to access the corresponding selenylated oxospiro-2,5-cyclohexadienones and spiro[5,5]trienones, respectively. The present three-component method offers the diverse substitutions on selenium involving two C-Se and one C-C bond formations.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

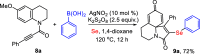

Similar articles
-
Electrochemical Selenylative Carbannulation of Biaryl Ynones to Seleno-Dibenzocycloheptenones/Spiro[5.5]Trienones.J Org Chem. 2021 Dec 3;86(23):17071-17081. doi: 10.1021/acs.joc.1c02182. Epub 2021 Nov 22. J Org Chem. 2021. PMID: 34808049
-
Expeditious Access to Spiro-Fused 2,5-Cyclohexadienones via Thio(seleno)cyanative ipso-Cyclization.J Org Chem. 2020 Dec 4;85(23):15521-15531. doi: 10.1021/acs.joc.0c02270. Epub 2020 Nov 22. J Org Chem. 2020. PMID: 33225702
-
Copper(II)-catalyzed oxidative ipso-annulation of N-arylpropiolamides and biaryl ynones with 1,3-diketones: construction of diketoalkyl spiro-trienones.Org Biomol Chem. 2022 Aug 31;20(34):6879-6889. doi: 10.1039/d2ob01282k. Org Biomol Chem. 2022. PMID: 35972321
-
Recent investigations into deborylative (thio-/seleno-) cyanation of aryl boronic acids.RSC Adv. 2024 Mar 19;14(13):9184-9199. doi: 10.1039/d4ra00487f. eCollection 2024 Mar 14. RSC Adv. 2024. PMID: 38505389 Free PMC article. Review.
-
ipso-Cyclization: an emerging tool for multifunctional spirocyclohexadienones.Org Biomol Chem. 2017 Apr 11;15(15):3130-3151. doi: 10.1039/c7ob00405b. Org Biomol Chem. 2017. PMID: 28338704 Review.
Cited by
-
Unified approach to synthesize diverse heterocyclics: a metal-free visible-light-promoted cyclization reaction to acquire sulfonylated spiro-trienones, coumarins and their derivatives.RSC Adv. 2025 Jul 8;15(29):23633-23642. doi: 10.1039/d5ra03553h. eCollection 2025 Jul 4. RSC Adv. 2025. PMID: 40630678 Free PMC article.
References
-
- Wu W.; Zhang L.; You S.-L. Catalytic Asymmetric Dearomatization (CADA) Reactions of Phenol and Aniline Derivatives. Chem. Soc. Rev. 2016, 45, 1570–1580. 10.1039/C5CS00356C. - DOI - PubMed
- Park H. B.; Kim Y.-J.; Lee J. K.; Lee K. R.; Kwon H. C. Spirobacillenes A and B, Unusual Spiro-cyclopentenones from Lysinibacillus fusiformis KMC003. Org. Lett. 2012, 14, 5002–5005. 10.1021/ol302115z. - DOI - PubMed
- D’yakonov V. A.; Trapeznikova O. A.; de Meijere A.; Dzhemilev U. M. Metal Complex Catalysis in the Synthesis of Spirocarbocycles. Chem. Rev. 2014, 114, 5775–5814. 10.1021/cr400291c. - DOI - PubMed
-
-
For selected reviews:
- Yang W. C.; Zhang M. M.; Feng J. G. Recent Advances in the Construction of Spiro Compounds via Radical Dearomatization. Adv. Synth. Catal. 2020, 362, 4446–4461. 10.1002/adsc.202000636. - DOI
- Vessally; Babazadeh M.; Didehban K.; Hosseinian A.; Edjlali L. Intramolecular ipso-Cyclization of N-Arylpropiolamides: A Novel and Straightforward Synthetic Approach for Azaspiro[4.5]decatrien-2-ones. Curr. Org. Chem. 2018, 22, 286–297. 10.2174/1385272821666170914111817. - DOI
- Reddy C. R.; Prajapti S. K.; Warudikar K.; Ranjan R.; Rao B. B. ipso-Cyclization: an emerging tool for multifunctional spirocyclohexadienones. Org. Biomol. Chem. 2017, 15, 3130–3151. 10.1039/C7OB00405B. - DOI - PubMed
-
-
- Yugandhar D.; Kuriakose S.; Nanubolu J. B.; Srivastava A. K. Synthesis of Alkaloid-Mimicking Tricyclic Skeletons by Diastereo and Regioselective Ugi/Ipso-Cyclization/Aza-Michael Cascade Reaction in One-Pot. Org. Lett. 2016, 18, 1040–1043. 10.1021/acs.orglett.6b00164. - DOI - PubMed
- Liang X.-W.; Zheng C.; You S.-L. Dearomatization through Halofunctionalization Reactions. Chem.—Eur. J. 2016, 22, 11918–11933. 10.1002/chem.201600885. - DOI - PubMed
-
- Nair A. M.; Shinde A. H.; Kumar S.; Volla C. M. R. Metal- Free Spirocyclization of N-Arylpropiolamides with Glyoxylic Acids: Access to Complex Azaspiro-Fused Tricycles. Chem. Commun. 2020, 56, 12367–12370. 10.1039/D0CC04800C. - DOI - PubMed
- Liu Y.; Wang Q.-L.; Zhou C.-S.; Xiong B.-Q.; Zhang P.-L.; Yang C.-a.; Tang K.-W. Visible-Light-Mediated Ipso-Carboacylation of Alkynes: Synthesis of 3-Acylspiro[4,5]Trienones from N-(P-Methoxyaryl)Propiolamides and Acyl Chlorides. J. Org. Chem. 2018, 83, 2210–2218. 10.1021/acs.joc.7b03104. - DOI - PubMed
- Jin D.-P.; Gao P.; Chen D.-Q.; Chen S.; Wang J.; Liu X.-Y.; Liang Y.-M. AgSCF3-Mediated Oxidative Trifluoromethythiolation of Alkynes with Dearomatization to Synthesize Scf3-Substituted Spiro[4,5]Trienones. Org. Lett. 2016, 18, 3486–3489. 10.1021/acs.orglett.6b01702. - DOI - PubMed
-
- Nair A. M.; Halder I.; Khan S.; Volla C. M. R. Metal Free Sulfonylative Spirocyclization of Alkenyl and Alkynyl Amides Via Insertion of Sulfur Dioxide. Adv. Synth. Catal. 2020, 362, 224–229. 10.1002/adsc.201901321. - DOI
- Wei W.; Cui H.-H.; Yang D.-S.; Yue H.-L.; He C.-L.; Zhang Y.-L.; Wang H. Visible-light-enabled spirocyclization of alkynes leading to 3-sulfonyl and 3- sulfenyl azaspiro[4,5]trienones. Green Chem. 2017, 19, 5608–5613. 10.1039/C7GC02330H. - DOI
LinkOut - more resources
Full Text Sources
