Density Functional Study of Size-Dependent Hydrogen Adsorption on Ag n Cr (n = 1-12) Clusters
- PMID: 36312417
- PMCID: PMC9607664
- DOI: 10.1021/acsomega.2c04107
Density Functional Study of Size-Dependent Hydrogen Adsorption on Ag n Cr (n = 1-12) Clusters
Abstract
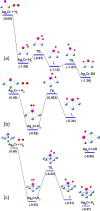
Increasing interest has been paid for hydrogen adsorption on atomically controlled nanoalloys due to their potential applications in catalytic processes and energy storage. In this work, we investigate the interaction of H2 with small-sized Ag n Cr (n = 1-12) using density functional theory calculations. It is found that the cluster structures are preserved during the adsorption of H2 either molecularly or dissociatively. Ag3Cr-H2, Ag6Cr-H2, and Ag9Cr-H2 clusters are identified to be relatively more stable from computed binding energies and second-order energy difference. The dissociation of adsorbed H2 on Ag2Cr, Ag3Cr, Ag6Cr, and Ag7Cr clusters is favored both thermodynamically and kinetically. The dissociative adsorption is unlikely to occur because of a considerable energy barrier before reaching the final state for Ag4Cr or due to energetic preferences for n = 1, 5, and 8-12 species. Comprehensive analysis shows that the geometric structure of clusters, the relative electronegativity, and the coordination number of the Cr impurity play a decisive role in determining the preferred adsorption configuration.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Vanbuel J.; Ferrari P.; Janssens E. Few-atom Cluster Model Systems for a Hydrogen Economy. Adv. Phys.: X 2020, 5, 1754132.10.1080/23746149.2020.1754132. - DOI
-
- Dawson J. K. Prospects for Hydrogen as an Energy Resource. Nature 1974, 249, 724.10.1038/249724a0. - DOI
-
- Niaz S.; Manzoor T.; Pandith A. H. Hydrogen Storage: Materials, Methods and Perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457.10.1016/j.rser.2015.05.011. - DOI
LinkOut - more resources
Full Text Sources
