Association study between hypothalamic functional connectivity, early nutrition, and glucose levels in healthy children aged 6 years: The COGNIS study follow-up
- PMID: 36313089
- PMCID: PMC9597646
- DOI: 10.3389/fnut.2022.935740
Association study between hypothalamic functional connectivity, early nutrition, and glucose levels in healthy children aged 6 years: The COGNIS study follow-up
Abstract
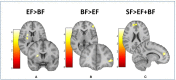
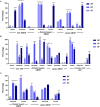
Breastfeeding (BF) is the gold standard in infant nutrition; knowing how it influences brain connectivity would help understand the mechanisms involved, which would help close the nutritional gap between infant formulas and breast milk. We analyzed potential long-term differences depending on the diet with an experimental infant formula (EF), compared to a standard infant formula (SF) or breastfeeding (BF) during the first 18 months of life on children's hypothalamic functional connectivity (FC) assessed at 6 years old. A total of 62 children participating in the COGNIS randomized clinical trial (Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT02094547) were included in this study. They were randomized to receive an SF (n = 22) or a bioactive nutrient-enriched EF (n = 20). BF children were also included as a control study group (BF: n = 20). Brain function was evaluated using functional magnetic resonance imaging (fMRI) and mean glucose levels were collected through a 24-h continuous glucose monitoring (CGM) device at 6 years old. Furthermore, nutrient intake was also analyzed during the first 18 months of life and at 6 years old through 3-day dietary intake records. Groups fed with EF and BF showed lower FC between the medial hypothalamus (MH) and the anterior cingulate cortex (ACC) in comparison with SF-fed children. Moreover, the BF children group showed lower FC between the MH and the left putamen extending to the middle insula, and higher FC between the MH and the inferior frontal gyrus (IFG) compared to the EF-fed children group. These areas are key regions within the salience network, which is involved in processing salience stimuli, eating motivation, and hedonic-driven desire to consume food. Indeed, current higher connectivity found on the MH-IFG network in the BF group was associated with lower simple sugars acceptable macronutrient distribution ranges (AMDRs) at 6 months of age. Regarding linoleic acid intake at 12 months old, a negative association with this network (MH-IFG) only in the BF group was found. In addition, BF children showed lower mean glucose levels compared to SF-fed children at 6 years old. Our results may point out a possible relationship between diet during the first 18 months of life and inclined proclivity for hedonic eating later in life.
Clinical trial registration: https://www.clinicaltrials.gov/, identifier NCT02094547.
Keywords: eating behavior; hypothalamus; long chain polyunsaturated fatty acids (LC-PUFAs); mean glucose levels; milk fat globule membrane (MFGM); neuroimaging; synbiotics.
Copyright © 2022 Diéguez, Nieto-Ruiz, Martín-Pérez, Sepúlveda-Valbuena, Herrmann, Jiménez, De-Castellar, Catena, García-Santos, Bermúdez and Campoy.
Conflict of interest statement
Authors JJ and RD-C are employees of Ordesa Laboratories, S.L. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

