Manganese Promoted (Bi)carbonate Hydrogenation and Formate Dehydrogenation: Toward a Circular Carbon and Hydrogen Economy
- PMID: 36313168
- PMCID: PMC9615124
- DOI: 10.1021/acscentsci.2c00723
Manganese Promoted (Bi)carbonate Hydrogenation and Formate Dehydrogenation: Toward a Circular Carbon and Hydrogen Economy
Abstract
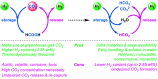
We report here a feasible hydrogen storage and release process by interconversion of readily available (bi)carbonate and formate salts in the presence of naturally occurring α-amino acids. These transformations are of interest for the concept of a circular carbon economy. The use of inorganic carbonate salts for hydrogen storage and release is also described for the first time. Hydrogenation of these substrates proceeds with high formate yields in the presence of specific manganese pincer catalysts and glutamic acid. Based on this, cyclic hydrogen storage and release processes with carbonate salts succeed with good H2 yields.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Brethomé F. M.; Williams N. J.; Seipp C. A.; Kidder M. K.; Custelcean R. Direct air capture of CO2 via aqueous-phase absorption and crystalline-phase release using concentrated solar power. Nat. Energy 2018, 3, 553–559. 10.1038/s41560-018-0150-z. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
