Transcriptomic analysis of the anti-inflammatory effect of Cordyceps militaris extract on acute gouty arthritis
- PMID: 36313318
- PMCID: PMC9614083
- DOI: 10.3389/fphar.2022.1035101
Transcriptomic analysis of the anti-inflammatory effect of Cordyceps militaris extract on acute gouty arthritis
Abstract
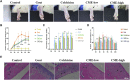
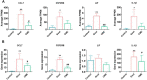
Background: Gouty arthritis (GA) is a common inflammatory disease that causes pain due to the deposition of monosodium urate (MSU) crystals into joints and surrounding tissues. Anti-inflammatory drugs have significant clinical anti-inflammatory and analgesic effects, but they have many side effects. Cordyceps militaris is an edible and medicinal fungus, and its extract (CME) has good anti-inflammatory and analgesic effects. This study aimed to investigate the anti-inflammatory effect of CME on GA and its underlying mechanism. Methods: The effect of CME on the expression of related inflammatory factors and histopathological changes in the MSU-induced acute inflammatory gout model in rats was studied by ELISA and HE, and its anti-inflammatory mechanism was analyzed by transcriptome combined with RT-qPCR. Results: CME significantly improved gait scores and joint swelling in GA rats, and reduced MSU-induced inflammatory cell infiltration. CME inhibited MSU-induced inflammatory responses by reducing the levels of pro-inflammatory factors TNF-α, IL-1β, IL-6, and Caspase-1 and increasing the anti-inflammatory factor IL-10. Transcriptome analysis showed that CME significantly altered inflammation-related cytokine pathways, and identified four major genes involved in regulation of inflammation, CCL7, CSF2RB, LIF, and IL-1β. In addition, RT-qPCR was performed to verify these differential genes. Conclusion: CME significantly alleviated the inflammatory progression of GA and ameliorated the onset of GA. The underlying mechanism may be related to triggering the cytokine-cytokine receptor interaction signaling pathway to inhibit the activation of the inflammasome and regulate the immune system. And it regulates the inflammatory response induced by MSU crystals through the genes CCL7, CSF2RB, and IL-1β.
Keywords: Cordyceps militaris extract; gouty arthritis; inflammation; monosodium urate; transcriptomics.
Copyright © 2022 Jiao, Liang, Liu, Li, Chen and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
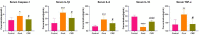

Similar articles
-
Curcumin ameliorates monosodium urate-induced gouty arthritis through Nod-like receptor 3 inflammasome mediation via inhibiting nuclear factor-kappa B signaling.J Cell Biochem. 2019 Apr;120(4):6718-6728. doi: 10.1002/jcb.27969. Epub 2018 Dec 28. J Cell Biochem. 2019. PMID: 30592318
-
Anti-inflammatory effects of traditional mixed extract of medicinal herbs (MEMH) on monosodium urate crystal-induced gouty arthritis.Chin J Nat Med. 2017 Aug;15(8):561-575. doi: 10.1016/S1875-5364(17)30084-5. Chin J Nat Med. 2017. PMID: 28939019
-
Dolichos falcata Klein attenuated the inflammation induced by monosodium urate crystals in vivo and in vitro.J Ethnopharmacol. 2013 Nov 25;150(2):545-52. doi: 10.1016/j.jep.2013.08.063. Epub 2013 Sep 20. J Ethnopharmacol. 2013. PMID: 24060409
-
P2X7R: a potential key regulator of acute gouty arthritis.Semin Arthritis Rheum. 2013 Dec;43(3):376-80. doi: 10.1016/j.semarthrit.2013.04.007. Epub 2013 Jun 17. Semin Arthritis Rheum. 2013. PMID: 23786870 Review.
-
Gut-immunity-joint axis: a new therapeutic target for gouty arthritis.Front Pharmacol. 2024 Feb 23;15:1353615. doi: 10.3389/fphar.2024.1353615. eCollection 2024. Front Pharmacol. 2024. PMID: 38464719 Free PMC article. Review.
Cited by
-
Study on the Underlying Mechanism of Yinhua Gout Granules in the Treatment of Gouty Arthritis by Integrating Transcriptomics and Network Pharmacology.Drug Des Devel Ther. 2024 Jul 19;18:3089-3112. doi: 10.2147/DDDT.S475442. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39050804 Free PMC article.
-
Unique Bioactives from Zombie Fungus (Cordyceps) as Promising Multitargeted Neuroprotective Agents.Nutrients. 2023 Dec 27;16(1):102. doi: 10.3390/nu16010102. Nutrients. 2023. PMID: 38201932 Free PMC article. Review.
-
Mesenchymal stem cells: a novel therapeutic approach for feline inflammatory bowel disease.Stem Cell Res Ther. 2024 Nov 9;15(1):409. doi: 10.1186/s13287-024-04038-y. Stem Cell Res Ther. 2024. PMID: 39522034 Free PMC article.
-
Gut microbiota and blood biomarkers in IBD-Related arthritis: insights from mendelian randomization.Sci Rep. 2025 Jan 2;15(1):514. doi: 10.1038/s41598-024-84116-6. Sci Rep. 2025. PMID: 39747467 Free PMC article.
-
Immunopharmacological Insights into Cordyceps spp.: Harnessing Therapeutic Potential for Sepsis.Curr Pharm Des. 2025;31(11):823-842. doi: 10.2174/0113816128326301240920040036. Curr Pharm Des. 2025. PMID: 39694962 Review.
References
-
- Andrés M., FrancéS R., Pascual E. (2015). SAT0328 uric acid enhances monosodium urate induced pro-inflammatory response in gouty patients: A basic and translational research study. Ann. Rheum. Dis. 74 (2), 777.2–8. 10.1136/annrheumdis-2015-eular.1958 - DOI
-
- Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., et al. (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183 (2), 787–791. 10.4049/jimmunol.0901363 - DOI - PMC - PubMed
-
- Begley C. G., Lopez A. F., Nicola N. A., Warren D. J., Vadas M. A., Sanderson C. J., et al. (1986). Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: A rapid and sensitive microassay for colony-stimulating factors. Blood 68 (1), 162–166. 10.1182/blood.v68.1.162.162 - DOI - PubMed
-
- Ben-Baruch A., Xu L., Young P. R., Bengali K., Oppenheim J. J., Wang J. M. (1995). Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors. C-C CKR1, a receptor for macrophage inflammatory protein-1 alpha/Rantes, is also a functional receptor for MCP3. J. Biol. Chem. 270 (38), 22123–22128. 10.1074/jbc.270.38.22123 - DOI - PubMed
LinkOut - more resources
Full Text Sources

