An exploratory, open-label, randomized, multicenter trial of hachimijiogan for mild Alzheimer's disease
- PMID: 36313371
- PMCID: PMC9616163
- DOI: 10.3389/fphar.2022.991982
An exploratory, open-label, randomized, multicenter trial of hachimijiogan for mild Alzheimer's disease
Abstract
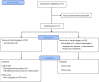
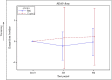
Background: Alzheimer's disease (AD) is a progressive neurodegeneration and is the most prevalent form of dementia. Intervention at an early stage is imperative. Although three acetylcholinesterase inhibitors (AChEIs) are currently approved for the treatment of mild AD, they are not sufficiently effective. Novel treatments for mild AD are of utmost importance. Objective: To assess the effectiveness of hachimijiogan (HJG), a traditional Japanese herbal medicine (Kampo), in the treatment of mild AD. Methods: This exploratory, open-label, randomized, multicenter trial enrolled patients with mild AD whose score on the Mini Mental State Examination (MMSE) was over 21points. All participants had been taking the same dosage of AChEI for more than 3 months. The participants were randomly assigned to an HJG group taking HJG extract 7.5 g/day in addition to AChEI or to a control group treated only with AChEI. The primary outcome was the change from baseline to 6 months post treatment initiation on the Alzheimer's Disease Assessment Scale-cognitive component- Japanese version(ADAS-Jcog). The secondary outcomes were change from baseline of the Instrumental Activity of Daily Life (IADL), Apathy scale, and Neuropsychiatric Inventory (NPI) -Q score. Results: Among the 77 enrollees, the data of 69(34 HJG and 35 control)were available for analysis. The difference in the change of ADAS-Jcog from baseline to 6 months of the HJG and control groups was 1.29 (90% Confidence interval (CI), -0.74 to 3.32 p = 0.293). In the subgroup analysis, the differences in the change from baseline to 3 and 6 months for women were 3.70 (90% CI ,0.50 to 6.91, p = 0.059) and 2.90 (90% CI,0.09 to 5.71, p = 0.090), respectively. For patients over 65 years, the difference at 3 months was 2.35 (90%CI, 0.01 to 4.68 p = 0.099). No significant differences were found between the HJG and control groups in IADL score, Apathy scale, or NPI-Q score. Conclusion: Although not conclusive, our data indicate that HJG has an adjuvant effect for acetylcholinesterase inhibitors and that it delays the deterioration of the cognitive dysfunction of mild Altzheimer's disease patients. Clinical Trial Registration: http://clinicaltrials.gov Japan Registry of clinical trials, identifier jRCTs 071190018.
Keywords: ADAS‐Jcog; Alzheimer’s disease; Kampo medicine; cognitive dysfunction; hachimijiogan.
Copyright © 2022 Kainuma, Ouma, Kawakatsu, Iritani, Yamashita, Ohara, Hirano, Suda, Hamano, Hieda, Yasui, Yoshiiwa, Shiota, Hironishi, Wada-Isoe, Sasabayashi, Yamasaki, Murata, Funakoshi, Hayashi, Shirafuji, Sasaki, Kajimoto, Mori, Suzuki, Ito, Ono and Tsuboi.
Conflict of interest statement
MK received a research grant from Tsumura that is not related to this study. Tsumura and Co. (Tokyo, Japan) provided TSUMURA hachimijiogan (TJ-7) Extract Granules for Ethical Use free of charge. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Department of Health and Human Services Public Health Service Food and Drug Administration US Food and Drug Administration (1989). Peripheral and central nervous system drugs advisory committee meeting. Rockville,MD,USA.
-
- Feldman H., Gauthier S., Hecker J., Vellas B., Emir B., Mastey V., et al. (2006). Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer's disease and the effect on caregiver burden. J. Am. Geriatr. Soc. 51, 737–744. 10.1046/j.1365-2389.2003.51260.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

