Increased Aurora B expression reduces substrate phosphorylation and induces chromosomal instability
- PMID: 36313574
- PMCID: PMC9606593
- DOI: 10.3389/fcell.2022.1018161
Increased Aurora B expression reduces substrate phosphorylation and induces chromosomal instability
Abstract
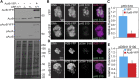
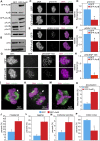
Increased Aurora B protein expression, which is common in cancers, is expected to increase Aurora B kinase activity, yielding elevated phosphorylation of Aurora B substrates. In contrast, here we show that elevated expression of Aurora B reduces phosphorylation of six different Aurora B substrates across three species and causes defects consistent with Aurora B inhibition. Complexes of Aurora B and its binding partner INCENP autophosphorylate in trans to achieve full Aurora B activation. Increased expression of Aurora B mislocalizes INCENP, reducing the local concentration of Aurora B:INCENP complexes at the inner centromere/kinetochore. Co-expression of INCENP rescues Aurora B kinase activity and mitotic defects caused by elevated Aurora B. However, INCENP expression is not elevated in concert with Aurora B in breast cancer, and increased expression of Aurora B causes resistance rather than hypersensitivity to Aurora B inhibitors. Thus, increased Aurora B expression reduces, rather than increases, Aurora B kinase activity.
Keywords: CIN; aurora kinase inhibitor; mitosis; mitotic checkpoint; spindle assembly checkpoint.
Copyright © 2022 Britigan, Wan, Sam, Copeland, Lasek, Hrycyniak, Wang, Audhya, Burkard, Roopra and Weaver.
Conflict of interest statement
M.E.B is a member of the Medical advisory board of Strata Oncology and receives Research funding from Abbvie, Genentech, Puma, Arcus, Apollomics, Loxo Oncology/Lilly, Seagen and Elevation Oncology. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Switching of INCENP paralogs controls transitions in mitotic chromosomal passenger complex functions.Cell Cycle. 2019 Sep;18(17):2006-2025. doi: 10.1080/15384101.2019.1634954. Epub 2019 Jul 15. Cell Cycle. 2019. PMID: 31306061 Free PMC article.
-
Aurora B kinase activity-dependent and -independent functions of the chromosomal passenger complex in regulating sister chromatid cohesion.J Biol Chem. 2019 Feb 8;294(6):2021-2035. doi: 10.1074/jbc.RA118.005978. Epub 2018 Dec 6. J Biol Chem. 2019. PMID: 30523151 Free PMC article.
-
Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis.Mol Biol Cell. 2003 Aug;14(8):3325-41. doi: 10.1091/mbc.e02-11-0769. Epub 2003 May 29. Mol Biol Cell. 2003. PMID: 12925766 Free PMC article.
-
Mitotic kinases: the key to duplication, segregation, and cytokinesis errors, chromosomal instability, and oncogenesis.Pharmacol Ther. 2006 Sep;111(3):974-84. doi: 10.1016/j.pharmthera.2006.02.006. Epub 2006 Apr 17. Pharmacol Ther. 2006. PMID: 16603252 Review.
-
The Molecular Mechanism of Aurora-B Regulating Kinetochore-Microtubule Attachment in Mitosis and Oocyte Meiosis.Cytogenet Genome Res. 2024;164(2):69-77. doi: 10.1159/000540588. Epub 2024 Jul 27. Cytogenet Genome Res. 2024. PMID: 39068909 Review.
Cited by
-
RUNX3 exerts tumor-suppressive role through inhibiting EXOSC4 expression.Funct Integr Genomics. 2024 May 17;24(3):103. doi: 10.1007/s10142-024-01363-6. Funct Integr Genomics. 2024. PMID: 38913281
-
Aurora B and INCENP co-overexpression severely disrupts mitosis and distinctly modifies the global transcriptional landscape.iScience. 2025 May 22;28(6):112731. doi: 10.1016/j.isci.2025.112731. eCollection 2025 Jun 20. iScience. 2025. PMID: 40530423 Free PMC article.
-
Optimal strategies for correcting merotelic chromosome attachments in anaphase.Proc Natl Acad Sci U S A. 2025 Feb 4;122(5):e2416459122. doi: 10.1073/pnas.2416459122. Epub 2025 Jan 30. Proc Natl Acad Sci U S A. 2025. PMID: 39883838 Free PMC article.
-
Double-checking chromosome segregation.J Cell Biol. 2023 May 1;222(5):e202301106. doi: 10.1083/jcb.202301106. Epub 2023 Apr 5. J Cell Biol. 2023. PMID: 37017932 Free PMC article. Review.
-
De-regulation of aurora kinases by oncogenic HPV; implications in cancer development and treatment.Tumour Virus Res. 2025 Jun;19:200314. doi: 10.1016/j.tvr.2025.200314. Epub 2025 Feb 7. Tumour Virus Res. 2025. PMID: 39923999 Free PMC article. Review.
References
-
- Adams R. R., Wheatleya S. P., Gouldsworthy A. M., Kandels-Lewis S. E., Carmena M., Smythe C., et al. (2000). INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 10 (17), 1075–1078. 10.1016/s0960-9822(00)00673-4 - DOI - PubMed
-
- Adams R. R., Maiato H., Earnshaw W. C., Carmena M. (2001). Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell. Biol. 153 (4), 865–880. 10.1083/jcb.153.4.865 - DOI - PMC - PubMed
-
- Azzariti A., Bocci G., Porcelli L., Fioravanti A., Sini P., Simone G. M., et al. (2011). Aurora B kinase inhibitor AZD1152: Determinants of action and ability to enhance chemotherapeutics effectiveness in pancreatic and colon cancer. Br. J. Cancer 104 (5), 769–780. 10.1038/bjc.2011.21 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

