High genotypic diversity of human papillomavirus among women in Cameroon: implications for vaccine effectiveness
- PMID: 36313603
- PMCID: PMC9596729
- DOI: 10.1016/j.ijregi.2022.09.014
High genotypic diversity of human papillomavirus among women in Cameroon: implications for vaccine effectiveness
Abstract
Background: The burden of human papillomavirus (HPV) is high in Cameroon, but knowledge on high-risk oncogenic HPV (HR-HPV) is limited. Our study sought to ascertain the HR-HPV genotypes circulating in Cameroon.
Methods: A cross-sectional study was conducted among non-vaccinated women in Cameroon. Detection of HR-HPV was performed by real-time PCR on cervico-vaginal swabs. Predictors of HR-HPV were determined following logistic regression analysis, with p < 0.05 considered statistically significant.
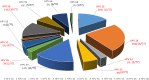
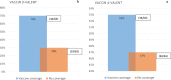
Results: In total, 364 women were enrolled, with a median age of 41 (34-50) years. Of these, 3.0% were smokers and 26.09% reported having more than three sexual partners. The overall HR-HPV positivity rate was 21.43% (95% CI 17.21-25.64). Predictors of HR-HPV were young age, i.e < 41 years (aOR (95% CI) 0.408 (0.194-0.862); p = 0.018), smoking (aOR 5.199 (1.314-20.575); p = 0.018), and having more than three sex partners (aOR: 2.335 (1.133-4.811); p = 0.022). Overall, 12 HR-HPV genotypes were identified, with 26.98% women coinfected with at least two HR-HPVs, including one case of a triple coinfection. According to to the circulating genotypes, potential vaccine effectiveness was 47% for the 4-valent vaccine and 70% for the 9-valent vaccine.
Conclusion: Within the Cameroonian context, at least one out of five women is likely to be an HR-HPV carrier, especially among young people, smokers, and those with multiple sexual partners. Importantly, HR-HPV infection is highly diversified, with vaccine efficacy ranging from about 47% (4-valent) to 70% (9-valent).
Keywords: Cameroon; HR-HPV; genotype; positivity rate; vaccination.
© 2022 The Author(s).
Figures
References
-
- Akom E., Venne S., Institut National de Santé Publique du Québec . Institut National de Santé Publique du Québec; 2003. L'infection au virus du papillome humain (VPH)http://www.inspq.qc.ca/publications/defaultlien.asp?E=p&NumPublication=1...
-
- Ali K.E., Mohammed I.A., Difabachew M.N., Demeke D.S., Haile T., Ten Hove R.-J., Kumssa T.H., Woldu Z.L., Haile E.L., Tullu K.D. Burden and genotype distribution of high-risk human papillomavirus infection and cervical cytology abnormalities at selected obstetrics and gynecology clinics of Addis Ababa. Ethiopia. BMC Cancer. 2019;19(1):768. doi: 10.1186/s12885-019-5953-1. - DOI - PMC - PubMed
-
- ANSM . Agence Nationale de Sécurité du Médicament et des Produits de Santé; 2006. Mise sur le marché du vaccin pour la prévention d'infections liées au virus papilloma.https://www.ansm.sante.fr/S-informer/Communiques-Communiques-Points-pres...
-
- Apgar B.S., Zoschnick L., Wright T.C. The 2001 Bethesda System terminology. American Family Physician. 2003;68(10):1992–1998. - PubMed
LinkOut - more resources
Full Text Sources