Artificial sleep-like up/down-states induce synaptic plasticity in cortical neurons from mouse brain slices
- PMID: 36313618
- PMCID: PMC9615418
- DOI: 10.3389/fncel.2022.948327
Artificial sleep-like up/down-states induce synaptic plasticity in cortical neurons from mouse brain slices
Abstract
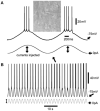
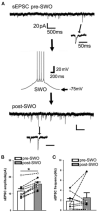
During non-rapid eye movement (NREM) sleep, cortical neuron activity alternates between a depolarized (firing, up-state) and a hyperpolarized state (down-state) coinciding with delta electroencephalogram (EEG) slow-wave oscillation (SWO, 0. 5-4 Hz) in vivo. Recently, we have found that artificial sleep-like up/down-states can potentiate synaptic strength in layer V cortical neurons ex vivo. Using mouse coronal brain slices, whole cell voltage-clamp recordings were made from layer V cortical pyramidal neurons to record spontaneous excitatory synaptic currents (sEPSCs) and inhibitory synaptic currents (sIPSCs). Artificial sleep-like up/down-states (as SWOs, 0.5 Hz, 10 min, current clamp mode) were induced by injecting sinusoidal currents into layer V cortical neurons. Baseline pre-SWO recordings were recorded for 5 min and post-SWO recordings for at least 25-30 min. Compared to pre-SWO sEPSCs or sIPSCs, post-SWO sEPSCs or sIPSCs in layer V cortical neurons exhibited significantly larger amplitudes and a higher frequency for 30 min. This finding suggests that both sEPSCs and sIPSCs could be potentiated in layer V cortical neurons by the low-level activity of SWOs, and sEPSCs and sIPSCs maintained a balance in layer V cortical neurons during pre- and post-SWO periods. Overall, this study presents an ex vivo method to show SWO's ability to induce synaptic plasticity in layer V cortical neurons, which may underlie sleep-related synaptic potentiation for sleep-related memory consolidation in vivo.
Keywords: brain slices; homeostatic synaptic plasticity; sleep; up/down-state; whole-cell recordings.
Copyright © 2022 Besing, St. John, Potesta, Gallagher and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

