Temporal radiographic and histological study of necrosis development in a mouse glioblastoma model
- PMID: 36313633
- PMCID: PMC9614031
- DOI: 10.3389/fonc.2022.993649
Temporal radiographic and histological study of necrosis development in a mouse glioblastoma model
Abstract
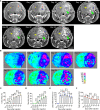
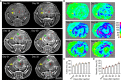
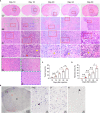
Tumor necrosis is a poor prognostic marker in glioblastoma (GBM) and a variety of other solid cancers. Accumulating evidence supports that necrosis could facilitate tumor progression and resistance to therapeutics. GBM necrosis is typically first detected by magnetic resonance imaging (MRI), after prominent necrosis has already formed. Therefore, radiological appearances of early necrosis formation and the temporal-spatial development of necrosis alongside tumor progression remain poorly understood. This knowledge gap leads to a lack of reliable radiographic diagnostic/prognostic markers in early GBM progression to detect necrosis. Recently, we reported an orthotopic xenograft GBM murine model driven by hyperactivation of the Hippo pathway transcriptional coactivator with PDZ-binding motif (TAZ) which recapitulates the extent of GBM necrosis seen among patients. In this study, we utilized this model to perform a temporal radiographic and histological study of necrosis development. We observed tumor tissue actively undergoing necrosis first appears more brightly enhancing in the early stages of progression in comparison to the rest of the tumor tissue. Later stages of tumor progression lead to loss of enhancement and unenhancing signals in the necrotic central portion of tumors on T1-weighted post-contrast MRI. This central unenhancing portion coincides with the radiographic and clinical definition of necrosis among GBM patients. Moreover, as necrosis evolves, two relatively more contrast-enhancing rims are observed in relationship to the solid enhancing tumor surrounding the central necrosis in the later stages. The outer more prominently enhancing rim at the tumor border probably represents the infiltrating tumor edge, and the inner enhancing rim at the peri-necrotic region may represent locally infiltrating immune cells. The associated inflammation at the peri-necrotic region was further confirmed by immunohistochemical study of the temporal development of tumor necrosis. Neutrophils appear to be the predominant immune cell population in this region as necrosis evolves. This study shows central, brightly enhancing areas associated with inflammation in the tumor microenvironment may represent an early indication of necrosis development in GBM.
Keywords: Glioblastoma; MRI; TAZ; magnetic resonance imaging; mouse model; tumor necrosis.
Copyright © 2022 Yee, Wang, Chih, Aregawi, Glantz, Zacharia, Thamburaj and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Hippo Pathway in Regulating Drug Resistance of Glioblastoma.Int J Mol Sci. 2021 Dec 14;22(24):13431. doi: 10.3390/ijms222413431. Int J Mol Sci. 2021. PMID: 34948224 Free PMC article. Review.
-
Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression.Nat Commun. 2020 Oct 27;11(1):5424. doi: 10.1038/s41467-020-19193-y. Nat Commun. 2020. PMID: 33110073 Free PMC article.
-
Quantitative T1-mapping detects cloudy-enhancing tumor compartments predicting outcome of patients with glioblastoma.Cancer Med. 2017 Jan;6(1):89-99. doi: 10.1002/cam4.966. Epub 2016 Nov 28. Cancer Med. 2017. PMID: 27891815 Free PMC article.
-
YAP/TAZ Transcriptional Coactivators Create Therapeutic Vulnerability to Verteporfin in EGFR-mutant Glioblastoma.Clin Cancer Res. 2021 Mar 1;27(5):1553-1569. doi: 10.1158/1078-0432.CCR-20-0018. Epub 2020 Nov 10. Clin Cancer Res. 2021. PMID: 33172899 Free PMC article.
-
Radiological features of infantile glioblastoma and desmoplastic infantile tumors: British Columbia's Children's Hospital experience.J Neurosurg Pediatr. 2015 Aug;16(2):119-25. doi: 10.3171/2014.10.PEDS13634. Epub 2015 May 8. J Neurosurg Pediatr. 2015. PMID: 25955808 Review.
Cited by
-
LC3-associated phagocytosis of neutrophils triggers tumor ferroptotic cell death in glioblastoma.EMBO J. 2024 Jul;43(13):2582-2605. doi: 10.1038/s44318-024-00130-4. Epub 2024 May 28. EMBO J. 2024. PMID: 38806658 Free PMC article.
-
Procyanidin B1 Promotes PSMC3-NRF2 Ubiquitination to Induce Ferroptosis in Glioblastoma.Phytother Res. 2024 Dec;38(12):5583-5597. doi: 10.1002/ptr.8328. Epub 2024 Sep 18. Phytother Res. 2024. PMID: 39293861 Free PMC article.
-
Tumor location, genomic alterations, and radiomic features as predictors of survival in glioblastoma: a Multi-Modal analysis.Neuroradiology. 2025 Aug 12. doi: 10.1007/s00234-025-03742-7. Online ahead of print. Neuroradiology. 2025. PMID: 40794131
-
Transcriptomic HEPN1 signatures predict treatment response in low grade glioma.Discov Oncol. 2025 Aug 15;16(1):1561. doi: 10.1007/s12672-025-03395-1. Discov Oncol. 2025. PMID: 40815398 Free PMC article.
-
Ferroptosis in glioma therapy: advancements in sensitizing strategies and the complex tumor-promoting roles.Brain Res. 2024 Oct 1;1840:149045. doi: 10.1016/j.brainres.2024.149045. Epub 2024 May 29. Brain Res. 2024. PMID: 38821335 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

