Prognostic role of HPV integration status and molecular profile in advanced anal carcinoma: An ancillary study to the epitopes-HPV02 trial
- PMID: 36313663
- PMCID: PMC9614213
- DOI: 10.3389/fonc.2022.941676
Prognostic role of HPV integration status and molecular profile in advanced anal carcinoma: An ancillary study to the epitopes-HPV02 trial
Abstract
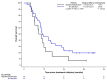
Squamous Cell Carcinoma of the Anal canal (SCCA) is a rare disease associated with a Human Papillomavirus (HPV) infection in most cases, predominantly the HPV16 genotype. About 15% of SCCA are diagnosed in metastatic stage and some will relapse after initial chemoradiotherapy (CRT). Treatment of patients by Docetaxel, Cisplatin and 5-fluorouracil (DCF) has been recently shown to improve their complete remission and progression-free survival. The aim of this retrospective study was to explore the impact of HPV infection, HPV DNA integration, TERT promoter mutational status and somatic mutations of oncogenes on both progression-free (PFS) and overall survivals (OS) of patients treated by DCF. Samples obtained from 49 patients included in the Epitopes-HPV02 clinical trial, diagnosed with metastatic or non-resectable local recurrent SCCA treated by DCF, were used for analyses. Median PFS and OS were not associated with HPV status. Patients with episomal HPV had an improved PFS compared with SCCA patients with integrated HPV genome (p=0.07). TERT promoter mutations were rarely observed and did not specifically distribute in a subset of SCCA and did not impact DCF efficacy. Among the 42 genes investigated, few gene alterations were observed, and were in majority amplifications (68.4%), but none were significantly correlated to PFS. As no biomarker is significantly associated with patients' survival, it prompts us to include every patient failing CRT or with metastatic disease in DCF strategy.
Keywords: HPV integration; NGS - next generation sequencing; SCCA; Somatic mutation analysis; TERT promoter mutation.
Copyright © 2022 Debernardi, Meurisse, Prétet, Guenat, Monnien, Spehner, Vienot, Roncarati, André, Abramowitz, Molimard, Mougin, Herfs, Kim and Borg.
Conflict of interest statement
CB has received a research grant from companies Bayer and Roche, and was an advisory board member of Bayer, MSD and Pierre Fabre companies. None of them had a role in the study design, analysis, or interpretation of the results. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol (2017) 46(3):924−38. doi: 1093/ije/dyw276 - PubMed
-
- Gunderson LL, Winter KA, Ajani JA, Pedersen JE, Moughan J, Benson AB, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: Survival, relapse, and colostomy failure with concurrent chemoradiation involving Fluorouracil/Mitomycin versus Fluorouracil/Cisplatin. J Clin Oncol (2012) 30(35):4344−51. doi: 10.1200/JCO.2012.43.8085 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

