Spontaneous preterm birth: Involvement of multiple feto-maternal tissues and organ systems, differing mechanisms, and pathways
- PMID: 36313741
- PMCID: PMC9606232
- DOI: 10.3389/fendo.2022.1015622
Spontaneous preterm birth: Involvement of multiple feto-maternal tissues and organ systems, differing mechanisms, and pathways
Abstract
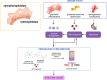
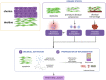
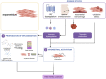
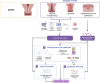
Survivors of preterm birth struggle with multitudes of disabilities due to improper in utero programming of various tissues and organ systems contributing to adult-onset diseases at a very early stage of their lives. Therefore, the persistent rates of low birth weight (birth weight < 2,500 grams), as well as rates of neonatal and maternal morbidities and mortalities, need to be addressed. Active research throughout the years has provided us with multiple theories regarding the risk factors, initiators, biomarkers, and clinical manifestations of spontaneous preterm birth. Fetal organs, like the placenta and fetal membranes, and maternal tissues and organs, like the decidua, myometrium, and cervix, have all been shown to uniquely respond to specific exogenous or endogenous risk factors. These uniquely contribute to dynamic changes at the molecular and cellular levels to effect preterm labor pathways leading to delivery. Multiple intervention targets in these different tissues and organs have been successfully tested in preclinical trials to reduce the individual impacts on promoting preterm birth. However, these preclinical trial data have not been effectively translated into developing biomarkers of high-risk individuals for an early diagnosis of the disease. This becomes more evident when examining the current global rate of preterm birth, which remains staggeringly high despite years of research. We postulate that studying each tissue and organ in silos, as how the majority of research has been conducted in the past years, is unlikely to address the network interaction between various systems leading to a synchronized activity during either term or preterm labor and delivery. To address current limitations, this review proposes an integrated approach to studying various tissues and organs involved in the maintenance of normal pregnancy, promotion of normal parturition, and more importantly, contributions towards preterm birth. We also stress the need for biological models that allows for concomitant observation and analysis of interactions, rather than focusing on these tissues and organ in silos.
Keywords: early delivery; inflammation; parturition; pregnancy; premature labor; preterm birth.
Copyright © 2022 Vidal, Lintao, Severino, Tantengco and Menon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

