Myocardial protection of S-nitroso-L-cysteine in diabetic cardiomyopathy mice
- PMID: 36313766
- PMCID: PMC9602402
- DOI: 10.3389/fendo.2022.1011383
Myocardial protection of S-nitroso-L-cysteine in diabetic cardiomyopathy mice
Abstract
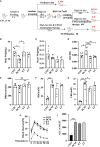
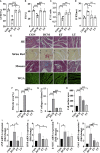
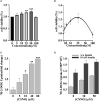
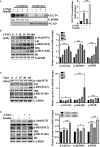
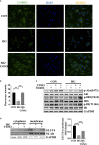
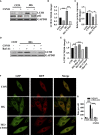
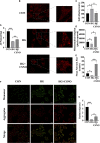
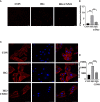
Diabetic cardiomyopathy (DCM) is a severe complication of diabetes mellitus that is characterized by aberrant myocardial structure and function and is the primary cause of heart failure and death in diabetic patients. Endothelial dysfunction plays an essential role in diabetes and is associated with an increased risk of cardiovascular events, but its role in DCM is unclear. Previously, we showed that S-nitroso-L-cysteine(CSNO), an endogenous S-nitrosothiol derived from eNOS, inhibited the activity of protein tyrosine phosphatase 1B (PTP1B), a critical negative modulator of insulin signaling. In this study, we reported that CSNO treatment induced cellular insulin-dependent and insulin-independent glucose uptake. In addition, CSNO activated insulin signaling pathway and promoted GLUT4 membrane translocation. CSNO protected cardiomyocytes against high glucose-induced injury by ameliorating excessive autophagy activation, mitochondrial impairment and oxidative stress. Furthermore, nebulized CSNO improved cardiac function and myocardial fibrosis in diabetic mice. These results suggested a potential site for endothelial modulation of insulin sensitivity and energy metabolism in the development of DCM. Data from these studies will not only help us understand the mechanisms of DCM, but also provide new therapeutic options for treatment.
Keywords: S-nitrosothiols; S-nitrosylation; diabetic cardiomyopathy; glucose uptake; insulin signaling pathway.
Copyright © 2022 Peng, Zhu, Huo, Shi, Jiang, Peng, Wang, Jiang, Guo, Men, Huang, Wang, Lv, Lin and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843 - DOI - PubMed
-
- Gulsin GS, Brady EM, Swarbrick DJ, Athithan L, Henson J, Baldry E, et al. . Rationale, design and study protocol of the randomised controlled trial: Diabetes interventional assessment of slimming or training tO lessen inconspicuous cardiovascular dysfunction (the DIASTOLIC study). BMJ Open (2019) 9:e023207. doi: 10.1136/bmjopen-2018-023207 - DOI - PMC - PubMed
-
- Li T, Ma X, Fedotov D, Kjaerulff L, Frydenvang K, Coriani S, et al. . Structure elucidation of prenyl- and geranyl-substituted coumarins in gerbera piloselloides by NMR spectroscopy, electronic circular dichroism calculations, and single crystal X-ray crystallography. Mol (Basel Switzerland) (2020) 25:1706. doi: 10.3390/molecules25071706 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

