Lack of placental neurosteroid alters cortical development and female somatosensory function
- PMID: 36313771
- PMCID: PMC9606442
- DOI: 10.3389/fendo.2022.972033
Lack of placental neurosteroid alters cortical development and female somatosensory function
Abstract
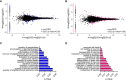
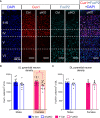
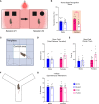
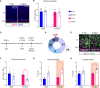
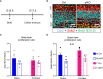
Placental endocrine function is essential to fetal brain development. Placental hormones include neurosteroids such as allopregnanolone (ALLO), a regulator of neurodevelopmental processes via positive allosteric modulation of the GABAA receptor (GABAA-R). Using a mouse model (plKO) in which the gene encoding the ALLO synthesis enzyme is specifically deleted in trophoblasts, we previously showed that placental ALLO insufficiency alters cerebellar white matter development and leads to male-specific autistic-like behavior. We now demonstrate that the lack of placental ALLO causes female-predominant alterations of cortical development and function. Placental ALLO insufficiency disrupts cell proliferation in the primary somatosensory cortex (S1) in a sex-linked manner. Early changes are seen in plKO embryos of both sexes, but persist primarily in female offspring after birth. Adolescent plKO females show significant reduction in pyramidal neuron density, as well as somatosensory behavioral deficits as compared with plKO males and control littermates. Assessment of layer-specific markers in human postmortem cortices suggests that preterm infants may also have female-biased abnormalities in cortical layer specification as compared with term infants. This study establishes a novel and fundamental link between placental function and sex-linked long-term neurological outcomes, emphasizing the importance of the growing field of neuroplacentology.
Keywords: GABAA receptor (GABAAR); allopregnanolone (3α,5α-THP); neuroplacentology; placenta; postmortem human brain; preterm birth; somatosensory cortex (S1).
Copyright © 2022 Bakalar, O’Reilly, Lacaille, Salzbank, Ellegood, Lerch, Sasaki, Imamura, Hashimoto-Torii, Vacher and Penn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Pasca AM, Penn AA. The placenta: The lost neuroendocrine organ. NeoReviews (2010) 11(2):e64–377. doi: 10.1542/neo.11-2-e64 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

