Systematic Review of Diagnostic Accuracy of DiaSorin Liaison SARS-CoV-2 Antigen Immunoassay
- PMID: 36313904
- PMCID: PMC9562478
Systematic Review of Diagnostic Accuracy of DiaSorin Liaison SARS-CoV-2 Antigen Immunoassay
Abstract
Background: Quantification of SARS-CoV-2 antigens by means of rapid, high-throughput and fully-automated techniques has been proposed as a feasible alternative to overcome the current shortage of resources for routine molecular diagnostics. To this end, we provide here a systematic review of diagnostic accuracy of DiaSorin Liaison SARS-CoV-2 antigen immunoassay.
Methods: An electronic search was conduced in Medline and Scopus, with no language or date restrictions (up to January 20, 2022), for identifying all published studies articles in which the diagnostic performance of the DiaSorin Liaison SARS-CoV-2 antigen immunoassay was compared with molecular diagnostic techniques.
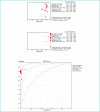
Results: The electronic search identified a final number of 11 studies, totalling 4449 oro- and naso-pharyngeal specimens. The pooled diagnostic sensitivity, specificity and area under the curve (AUC) of the Liaison SARS-CoV-2 antigen immunoassay in all samples were 0.51 (95%CI, 0.49-0.54), 1.00 (95%CI, 1.00-1.00) and 0.994 (95%CI, 0.990-0.998), respectively, whilst the overall concordance with molecular diagnostics was 82.1%. The pooled diagnostic sensitivity, specificity and AUC of the Liaison SARS-CoV-2 antigen immunoassay in specimens with high viral load (i.e., cycle threshold values <25-30) were 0.79 (95%CI, 0.75-0.82), 1.00 (95%CI, 0.99-1.00) and 0.911 (95%CI, 0.879-0.943), respectively, whilst the overall concordance with molecular diagnostics in such samples increased to 94.2%.
Conclusion: The results of this systematic literature review suggest that there is sufficient accuracy of the DiaSorin Liaison SARS-CoV-2 antigen immunoassay in samples with high viral loads that would enable its reliable usage for identifying superspreaders, who are responsible for the vast majority of transmission events.
Keywords: COVID-19; SARS-CoV-2; antigen; diagnosis; immunoassay.
Copyright © 2022 International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). All rights reserved.
Conflict of interest statement
None
Figures

Similar articles
-
Clinical Assessment of the DiaSorin LIAISON SARS-CoV-2 Ag Chemiluminescence Immunoassay.EJIFCC. 2021 Jun 29;32(2):216-223. eCollection 2021 Jun. EJIFCC. 2021. PMID: 34421491 Free PMC article.
-
Diagnostic performance of the fully automated Roche Elecsys SARS-CoV-2 antigen electrochemiluminescence immunoassay: a pooled analysis.Clin Chem Lab Med. 2022 Feb 3;60(5):655-661. doi: 10.1515/cclm-2022-0053. Print 2022 Apr 26. Clin Chem Lab Med. 2022. PMID: 35114742
-
Critical literature review and pooled analysis of diagnostic accuracy of Ortho VITROS SARS-CoV-2 antigen test for diagnosing acute SARS-CoV-2 infections.J Med Biochem. 2022 Oct 15;41(4):540-548. doi: 10.5937/jomb0-36107. J Med Biochem. 2022. PMID: 36381069 Free PMC article.
-
Fujirebio Lumipulse SARS-CoV-2 antigen immunoassay: pooled analysis of diagnostic accuracy.Diagnosis (Berl). 2022 Mar 15;9(2):149-156. doi: 10.1515/dx-2022-0021. Diagnosis (Berl). 2022. PMID: 35287253 Review.
-
LumiraDX SARS-CoV-2 Antigen Test for Diagnosing Acute SARS-CoV-2 Infection: Critical Literature Review and Meta-Analysis.Diagnostics (Basel). 2022 Apr 11;12(4):947. doi: 10.3390/diagnostics12040947. Diagnostics (Basel). 2022. PMID: 35453996 Free PMC article. Review.
References
-
- Lippi G, Mattiuzzi C, Henry BM. Updated picture of SARS-CoV-2 variants and mutations. Diagnosis (Berl) 2021;9: 11-17. - PubMed
-
- John Hopkins University. Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Last accessed, July 27, 2022.
-
- American Association of Clinical Chemistry. Coronavirus Testing Survey. Available at: https://www.aacc.org/science-and-research/covid-19-resources/aacc-covid-.... Last accessed: January 20, 2022.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
