Imbalance of NRG1-ERBB2/3 signalling underlies altered myelination in Charcot-Marie-Tooth disease 4H
- PMID: 36314052
- PMCID: PMC10151191
- DOI: 10.1093/brain/awac402
Imbalance of NRG1-ERBB2/3 signalling underlies altered myelination in Charcot-Marie-Tooth disease 4H
Abstract
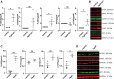
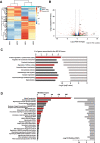
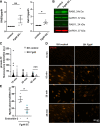
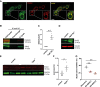
Charcot-Marie-Tooth (CMT) disease is one of the most common inherited neurological disorders, affecting either axons from the motor and/or sensory neurons or Schwann cells of the peripheral nervous system (PNS) and caused by more than 100 genes. We previously identified mutations in FGD4 as responsible for CMT4H, an autosomal recessive demyelinating form of CMT disease. FGD4 encodes FRABIN, a GDP/GTP nucleotide exchange factor, particularly for the small GTPase Cdc42. Remarkably, nerves from patients with CMT4H display excessive redundant myelin figures called outfoldings that arise from focal hypermyelination, suggesting that FRABIN could play a role in the control of PNS myelination. To gain insights into the role of FGD4/FRABIN in Schwann cell myelination, we generated a knockout mouse model (Fgd4SC-/-), with conditional ablation of Fgd4 in Schwann cells. We show that the specific deletion of FRABIN in Schwann cells leads to aberrant myelination in vitro, in dorsal root ganglia neuron/Schwann cell co-cultures, as well as in vivo, in distal sciatic nerves from Fgd4SC-/- mice. We observed that those myelination defects are related to an upregulation of some interactors of the NRG1 type III/ERBB2/3 signalling pathway, which is known to ensure a proper level of myelination in the PNS. Based on a yeast two-hybrid screen, we identified SNX3 as a new partner of FRABIN, which is involved in the regulation of endocytic trafficking. Interestingly, we showed that the loss of FRABIN impairs endocytic trafficking, which may contribute to the defective NRG1 type III/ERBB2/3 signalling and myelination. Using RNA-Seq, in vitro, we identified new potential effectors of the deregulated pathways, such as ERBIN, RAB11FIP2 and MAF, thereby providing cues to understand how FRABIN contributes to proper ERBB2 trafficking or even myelin membrane addition through cholesterol synthesis. Finally, we showed that the re-establishment of proper levels of the NRG1 type III/ERBB2/3 pathway using niacin treatment reduces myelin outfoldings in nerves of CMT4H mice. Overall, our work reveals a new role of FRABIN in the regulation of NRG1 type III/ERBB2/3 NRG1signalling and myelination and opens future therapeutic strategies based on the modulation of the NRG1 type III/ERBB2/3 pathway to reduce CMT4H pathology and more generally other demyelinating types of CMT disease.
Keywords: Charcot–Marie–Tooth; NRG1/ERBB2/3; endocytic trafficking; myelin outfoldings; niacin treatment.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Skre H. Genetic and clinical aspects of Charcot–Marie–Tooth’s disease. Clin Genet. 1974;6:98–118. - PubMed
-
- Harding AE, Thomas PK. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain. 1980;103:259–280. - PubMed
-
- Pareyson D, Scaioli V, Laura M. Clinical and electrophysiological aspects of Charcot–Marie–Tooth disease. Historical article review. Neuromolecular Med. 2006;8(1–2):3–22. - PubMed
-
- Pipis M, Rossor AM, Laura M, Reilly MM. Next-generation sequencing in Charcot–Marie–Tooth disease: Opportunities and challenges. Nat Rev Neurol. 2019;15:644–656. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

