Malignant teratoid intraocular ciliary body medulloepithelioma in a 5-year-old male with corresponding somatic copy number alteration profile of aqueous humor cell-free DNA
- PMID: 36314385
- PMCID: PMC9877122
- DOI: 10.1080/13816810.2022.2138457
Malignant teratoid intraocular ciliary body medulloepithelioma in a 5-year-old male with corresponding somatic copy number alteration profile of aqueous humor cell-free DNA
Abstract
Background: Intraocular, ciliary body, medulloepithelioma (CBME) is a rare tumor of the nonpigmented ciliary body epithelium, typically presenting in childhood. We describe a case of CBME.
Materials and methods: Ocular examination and imaging guided diagnostic and treatment decisions. Aqueous humor (AH) liquid biopsy was collected from the affected eye at eventual enucleation. Whole genome sequencing (WGS) was employed to determine somatic copy number alterations (SCNA) in AH cell-free DNA (cfDNA). Tumor sample was analyzed using various assays to evaluate for oncogenic mutations and SCNAs. Histopathology determined diagnosis.
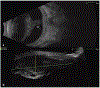
Results: A 5-year-old male with glaucoma and cataract in the left eye (OS) experienced worsening left eye pain and redness. There was no light perception OS and the eye was hypotonus. Anterior segment exam showed complete cataract and rubeosis iridis. Ocular B-scan ultrasound OS revealed an intraocular lesion with calcifications and retinal detachment. Orbital MRI suggested left globe hypercellularity. An infiltrative lesion involving the ciliary body was seen in the left eye on examination under anesthesia. Left eye enucleation was performed in the setting of pain, blindness, and tumor, with anterior chamber paracentesis for AH liquid biopsy collection. SCNA profile of AH cfDNA demonstrated loss of copy of chromosomes 4, 6, and 9. Tumor was negative for clinically significant mutations or SCNAs. Histopathology diagnosed malignant teratoid CBME.
Conclusions: We present a case of CBME and include the unique SCNA profile of AH cfDNA from the enucleated eye. This case suggests utility of AH liquid biopsy in distinguishing between differential diagnoses for intraocular mass lesions.
Keywords: Malignant teratoid medulloepithelioma; aqueous humor liquid biopsy; intraocular ciliary body medulloepithelioma; somatic copy number alteration profile.
Figures
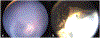
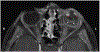
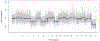

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
