Inhaled Carbon Dioxide Improves Neurological Outcomes by Downregulating Hippocampal Autophagy and Apoptosis in an Asphyxia-Induced Cardiac Arrest and Resuscitation Rat Model
- PMID: 36314493
- PMCID: PMC9673650
- DOI: 10.1161/JAHA.122.027685
Inhaled Carbon Dioxide Improves Neurological Outcomes by Downregulating Hippocampal Autophagy and Apoptosis in an Asphyxia-Induced Cardiac Arrest and Resuscitation Rat Model
Abstract
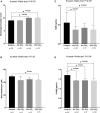
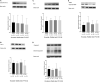
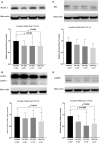
Background Protracted cerebral hypoperfusion following cardiac arrest (CA) may cause poor neurological recovery. We hypothesized that inhaled carbon dioxide (CO2) could augment cerebral blood flow (CBF) and improve post-CA neurological outcomes. Methods and Results After 6-minute asphyxia-induced CA and resuscitation, Wistar rats were randomly allocated to 4 groups (n=25/group) and administered with different inhaled CO2 concentrations, including control (0% CO2), 4% CO2, 8% CO2, and 12% CO2. Invasive monitoring was maintained for 120 minutes, and neurological outcomes were evaluated with neurological function score at 24 hours post-CA. After the 120-minute experiment, CBF was 242.3% (median; interquartile range, 221.1%-267.4%) of baseline in the 12% CO2 group while CBF fell to 45.8% (interquartile range, 41.2%-58.1%) of baseline in the control group (P<0.001). CBF increased along with increasing inhaled CO2 concentrations with significant linear trends (P<0.001). At 24 hours post-CA, compared with the control group (neurological function score, 9 [interquartile range, 8-9]), neurological recovery was significantly better in the 12% CO2 group (neurological function score, 10 [interquartile range, 9.8-10]) (P<0.001) while no survival difference was observed. Brain tissue malondialdehyde (P=0.02) and serum neuron-specific enolase (P=0.002) and S100β levels (P=0.002) were significantly lower in the 12% CO2 group. TUNEL (terminal deoxynucleotidyl transferase-mediated biotin-deoxyuridine triphosphate nick-end labeling)-positive cell densities in hippocampal CA1 (P<0.001) and CA3 (P<0.001) regions were also significantly reduced in the 12% CO2 group. Western blotting showed that beclin-1 (P=0.02), p62 (P=0.02), and LAMP2 (lysosome-associated membrane protein 2) (P=0.01) expression levels, and the LC3B-II:LC3B-I ratio (P=0.02) were significantly lower in the 12% CO2 group. Conclusions Administering inhaled CO2 augmented post-CA CBF, mitigated oxidative brain injuries, ameliorated neuronal injury, and downregulated apoptosis and autophagy, thereby improving neurological outcomes.
Keywords: apoptosis; autophagy; carbon dioxide; cardiac arrest; cerebral blood flow.
Figures



Similar articles
-
Neuroprotective Approaches for Brain Injury After Cardiac Arrest: Current Trends and Prospective Avenues.J Stroke. 2024 May;26(2):203-230. doi: 10.5853/jos.2023.04329. Epub 2024 May 30. J Stroke. 2024. PMID: 38836269 Free PMC article. Review.
-
Cerebral Blood Flow-Guided Manipulation of Arterial Blood Pressure Attenuates Hippocampal Apoptosis After Asphyxia-Induced Cardiac Arrest in Rats.J Am Heart Assoc. 2020 Jul 7;9(13):e016513. doi: 10.1161/JAHA.120.016513. Epub 2020 Jun 17. J Am Heart Assoc. 2020. PMID: 32552439 Free PMC article.
-
Hydrogen-rich saline improves survival and neurological outcome after cardiac arrest and cardiopulmonary resuscitation in rats.Anesth Analg. 2014 Aug;119(2):368-380. doi: 10.1213/ANE.0000000000000303. Anesth Analg. 2014. PMID: 24937348
-
[Comparison of brain injuries in rat cardiac arrest models induced by asphyxia and electrical stimulation].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020 Mar;32(3):336-340. doi: 10.3760/cma.j.cn121430-20200302-00204. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020. PMID: 32385999 Chinese.
-
[Effect of electroacupuncture at Baihui ameliorated neurologic deficit and hemodynamic stability in rat model of post-cardiac arrest syndrome].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022 Dec;34(12):1285-1290. doi: 10.3760/cma.j.cn121430-20210115-00068. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022. PMID: 36567584 Chinese.
Cited by
-
Neuroprotective Approaches for Brain Injury After Cardiac Arrest: Current Trends and Prospective Avenues.J Stroke. 2024 May;26(2):203-230. doi: 10.5853/jos.2023.04329. Epub 2024 May 30. J Stroke. 2024. PMID: 38836269 Free PMC article. Review.
-
Translational approach to assess brain injury after cardiac arrest in preclinical models: a narrative review.Intensive Care Med Exp. 2025 Jan 14;13(1):3. doi: 10.1186/s40635-024-00710-y. Intensive Care Med Exp. 2025. PMID: 39808393 Free PMC article. Review. No abstract available.
-
Effects of intrathecal administration of sodium nitroprusside and nicardipine on cerebral pial microcirculation, cortical tissue oxygenation, and electrocortical activity in the early post-resuscitation period in a porcine cardiac arrest model.PLoS One. 2025 Jan 29;20(1):e0313257. doi: 10.1371/journal.pone.0313257. eCollection 2025. PLoS One. 2025. PMID: 39879197 Free PMC article.
-
Stem cell-derived extracellular vesicles: novel therapeutics for cerebral injury following cardiac arrest and potential mechanisms.Cell Biosci. 2025 Jul 26;15(1):110. doi: 10.1186/s13578-025-01451-5. Cell Biosci. 2025. PMID: 40713691 Free PMC article. Review.
-
Optimal inhaled oxygen and carbon dioxide concentrations for post-cardiac arrest cerebral reoxygenation and neurological recovery.iScience. 2023 Nov 16;26(12):108476. doi: 10.1016/j.isci.2023.108476. eCollection 2023 Dec 15. iScience. 2023. PMID: 38187189 Free PMC article.
References
-
- Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, et al. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017 - DOI - PubMed
-
- Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, Carli P, Mira JP, Nolan J, Cariou A. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39:1972–1980. doi: 10.1007/s00134-013-3043-4 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

