Real-world evidence of subcutaneous treprostinil use in pulmonary arterial hypertension in Argentina
- PMID: 36314498
- PMCID: PMC9629562
- DOI: 10.1177/17534666221132735
Real-world evidence of subcutaneous treprostinil use in pulmonary arterial hypertension in Argentina
Abstract
Introduction: Pulmonary arterial hypertension is a progressive haemodynamic disease with high morbidity and mortality. Of the different treatments available, the prostacyclin analogues are the drugs of choice for high-risk patients, with treprostinil being the most commonly used drug in Argentina.
Methodology: The objective of this study is to perform a retrospective evaluation of the efficacy and safety of subcutaneous treprostinil in regular clinical practice in Argentina in 51 patients with pulmonary arterial hypertension after 12 months of follow-up.
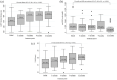
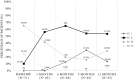
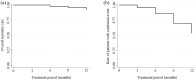
Results: The results showed that treatment with subcutaneous treprostinil is associated with a significant improvement in different clinical efficacy parameters: 65% reduction in advanced functional class (p < 0.0001), 130-m increase in the 6-min walk test (p < 0.0001), 65% reduction in the pro B-type natriuretic peptide value (-531 pg/dL; p < 0.0001), significant reduction of 15.7% in pulmonary vascular resistance [-1.3 wood units (WU); p < 0.0001], improved cardiac index with an increase of 16.7% (+0.4 L/min/m2; p = 0.002), as well as a high survival rate (92%) and a 44% incidence of combined events (mortality, heart failure, syncope and/or lung transplantation), without a significant increase in previously reported adverse events. The risk stratification evaluation according to ESC/ERS guidelines showed a significant decrease in the proportion of patients at high risk after the treatment period (p = 0.004).
Conclusions: These real-world results corroborate the efficacy and safety of subcutaneous treprostinil, even at high doses, and open up the possibility of improving its current use in clinical practice as a first-line therapy, especially in high-risk patient profiles.
Keywords: population registry; prostacyclin; prostanoids; pulmonary arterial hypertension; retrospective studies; survival rate.
Conflict of interest statement
All authors declare that they have no conflicts of interest in the development of the study and manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. - PubMed
-
- McLaughlin VV, Gaine SP, Barst RJ, et al. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol 2003; 41: 293–299. - PubMed
-
- Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Española Cardiol (English Ed) 2016; 69: 177. - PubMed
-
- Lang I, Gomez-Sanchez M, Kneussl M, et al. Efficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertension. Chest 2006; 129: 1636–1643. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

