Enhanced Inactivation of Pseudoparticles Containing SARS-CoV-2 S Protein Using Magnetic Nanoparticles and an Alternating Magnetic Field
- PMID: 36314574
- PMCID: PMC9691609
- DOI: 10.1021/acsabm.2c00522
Enhanced Inactivation of Pseudoparticles Containing SARS-CoV-2 S Protein Using Magnetic Nanoparticles and an Alternating Magnetic Field
Abstract
Severe acute respiratory syndrome coronavirus 2's (SARS-CoV-2) rapid global spread has posed a significant threat to human health, and similar outbreaks could occur in the future. Developing effective virus inactivation technologies is critical to preventing and overcoming pandemics. The infection of SARS-CoV-2 depends on the binding of the spike glycoprotein (S) receptor binding domain (RBD) to the host cellular surface receptor angiotensin-converting enzyme 2 (ACE2). If this interaction is disrupted, SARS-CoV-2 infection could be inhibited. Magnetic nanoparticle (MNP) dispersions exposed to an alternating magnetic field (AMF) possess the unique ability for magnetically mediated energy delivery (MagMED); this localized energy delivery and associated mechanical, chemical, and thermal effects are a possible technique for inactivating viruses. This study investigates the MNPs' effect on vesicular stomatitis virus pseudoparticles containing the SARS-CoV-2 S protein when exposed to AMF or a water bath (WB) with varying target steady-state temperatures (45, 50, and 55 °C) for different exposure times (5, 15, and 30 min). In comparison to WB exposures at the same temperatures, AMF exposures resulted in significantly greater inactivation in multiple cases. This is likely due to AMF-induced localized heating and rotation of MNPs. In brief, our findings demonstrate a potential strategy for combating the SARS-CoV-2 pandemic or future ones.
Keywords: COVID-19; SARS-CoV-2; alternating magnetic field; magnetic nanoparticles; virus inactivation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


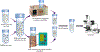
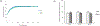

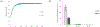
Similar articles
-
Optimized Pseudotyping Conditions for the SARS-COV-2 Spike Glycoprotein.J Virol. 2020 Oct 14;94(21):e01062-20. doi: 10.1128/JVI.01062-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32788194 Free PMC article.
-
Comparison of Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein Binding to ACE2 Receptors from Human, Pets, Farm Animals, and Putative Intermediate Hosts.J Virol. 2020 Jul 16;94(15):e00831-20. doi: 10.1128/JVI.00831-20. Print 2020 Jul 16. J Virol. 2020. PMID: 32404529 Free PMC article.
-
Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2.J Med Virol. 2020 Jun;92(6):595-601. doi: 10.1002/jmv.25726. Epub 2020 Mar 11. J Med Virol. 2020. PMID: 32100877 Free PMC article.
-
Potential use of the S-protein-Angiotensin converting enzyme 2 binding pathway in the treatment of coronavirus disease 2019.Front Public Health. 2022 Nov 28;10:1050034. doi: 10.3389/fpubh.2022.1050034. eCollection 2022. Front Public Health. 2022. PMID: 36518573 Free PMC article. Review.
-
Potential pathogenesis of severe acute respiratory syndrome coronavirus 2.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020 May 28;45(5):591-597. doi: 10.11817/j.issn.1672-7347.2020.200299. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 32879112 Review. Chinese, English.
Cited by
-
Magnetic Nanocomposites for the Remote Activation of Sulfate Radicals for the Removal of Rhodamine B.Nanomaterials (Basel). 2023 Mar 23;13(7):1151. doi: 10.3390/nano13071151. Nanomaterials (Basel). 2023. PMID: 37049245 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
