Constitutively active STING causes neuroinflammation and degeneration of dopaminergic neurons in mice
- PMID: 36314770
- PMCID: PMC9767458
- DOI: 10.7554/eLife.81943
Constitutively active STING causes neuroinflammation and degeneration of dopaminergic neurons in mice
Abstract
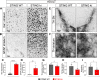
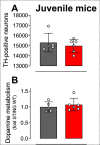
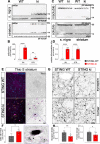
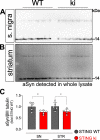
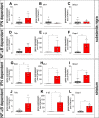
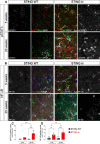
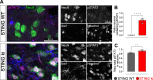
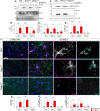
Stimulator of interferon genes (STING) is activated after detection of cytoplasmic dsDNA by cGAS (cyclic GMP-AMP synthase) as part of the innate immunity defence against viral pathogens. STING binds TANK-binding kinase 1 (TBK1). TBK1 mutations are associated with familial amyotrophic lateral sclerosis, and the STING pathway has been implicated in the pathogenesis of further neurodegenerative diseases. To test whether STING activation is sufficient to induce neurodegeneration, we analysed a mouse model that expresses the constitutively active STING variant N153S. In this model, we focused on dopaminergic neurons, which are particularly sensitive to stress and represent a circumscribed population that can be precisely quantified. In adult mice expressing N153S STING, the number of dopaminergic neurons was smaller than in controls, as was the density of dopaminergic axon terminals and the concentration of dopamine in the striatum. We also observed alpha-synuclein pathology and a lower density of synaptic puncta. Neuroinflammation was quantified by staining astroglia and microglia, by measuring mRNAs, proteins and nuclear translocation of transcription factors. These neuroinflammatory markers were already elevated in juvenile mice although at this age the number of dopaminergic neurons was still unaffected, thus preceding the degeneration of dopaminergic neurons. More neuroinflammatory markers were blunted in mice deficient for inflammasomes than in mice deficient for signalling by type I interferons. Neurodegeneration, however, was blunted in both mice. Collectively, these findings demonstrate that chronic activation of the STING pathway is sufficient to cause degeneration of dopaminergic neurons. Targeting the STING pathway could therefore be beneficial in Parkinson's disease and further neurodegenerative diseases.
Keywords: inflammasome; innate immunity; mouse; neurodegeneration; neuroinflammation; neuroscience; parkinson; synuclein.
Plain language summary
Neurodegenerative conditions such as Alzheimer’s and Parkinson’s diseases are characterised by neurons getting damaged and dying. Many factors contribute to this process, but few can be effectively controlled by therapies. Interestingly, previous studies have highlighted that inflammation, a process normally triggered by foreign agents or biological damage, is often associated with neurons degenerating. However, it is unclear whether these responses are the cause or the consequence of brain cell damage. In injured neurons, the genetic information normally contained inside a dedicated cellular compartment can start to leak into the surrounding parts of the cell. This damage triggers an inflammatory response through the STING pathway, a mechanism previously implicated in the onset of Parkinson’s disease. In these patients, the neurons that produce the signalling molecule dopamine start to die, leading to difficulty with movement. Whether STING can directly cause this neuronal loss remains unknown. To answer this question, Szegö, Malz et al. genetically engineered mice in which the STING pathway is permanently activated. The animals had fewer dopamine-producing neurons and accumulated harmful clumps of proteins; both these biological features are characteristic signs of Parkinson’s disease. Crucially, signs of inflammation were present before neurons started to show damage, suggesting that inflammatory responses could cause neurodegeneration. Further experiments revealed that STING triggers several molecular cascades; blocking one only of these pathways did not keep the neurons healthy. Neurodegenerative diseases are a growing concern around the world. The results from Szegö, Malz et al. suggest that preventing prolonged inflammatory may reduce the risk of neurodegeneration. If further research confirms these findings, in particular in humans, well-known treatments against inflammation could potentially become relevant to fight these conditions.
© 2022, Szego, Malz et al.
Conflict of interest statement
ES, LM, NB, AR, BF, HL No competing interests declared
Figures







References
-
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. The Journal of Biological Chemistry. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. - DOI - PubMed
-
- Balka KR, Louis C, Saunders TL, Smith AM, Calleja DJ, D’Silva DB, Moghaddas F, Tailler M, Lawlor KE, Zhan Y, Burns CJ, Wicks IP, Miner JJ, Kile BT, Masters SL, De Nardo D. Tbk1 and IKKε act redundantly to mediate STING-induced NF-κB responses in myeloid cells. Cell Reports. 2020;31:107492. doi: 10.1016/j.celrep.2020.03.056. - DOI - PubMed
-
- Bantle CM, Rocha SM, French CT, Phillips AT, Tran K, Olson KE, Bass TA, Aboellail T, Smeyne RJ, Tjalkens RB. Astrocyte inflammatory signaling mediates α-synuclein aggregation and dopaminergic neuronal loss following viral encephalitis. Experimental Neurology. 2021;346:113845. doi: 10.1016/j.expneurol.2021.113845. - DOI - PMC - PubMed
-
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappab activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. Journal of Immunology. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

