Infant Antibody Repertoires during the First Two Years of Influenza Vaccination
- PMID: 36314798
- PMCID: PMC9765176
- DOI: 10.1128/mbio.02546-22
Infant Antibody Repertoires during the First Two Years of Influenza Vaccination
Abstract
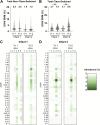
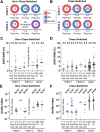
The first encounter with influenza virus biases later immune responses. This "immune imprinting," formerly from infection within a few years of birth, is in the United States now largely from immunization with a quadrivalent, split vaccine (IIV4 [quadrivalent inactivated influenza vaccine]). In a pilot study of IIV4 imprinting, we used single-cell cultures, next-generation sequencing, and plasma antibody proteomics to characterize the primary antibody responses to influenza in two infants during their first 2 years of seasonal influenza vaccination. One infant, who received only a single vaccination in year 1, contracted an influenza B virus (IBV) infection between the 2 years, allowing us to compare imprinting by infection and vaccination. That infant had a shift in hemagglutinin (HA)-reactive B cell specificity from largely influenza A virus (IAV) specific in year 1 to IBV specific in year 2, both before and after the year 2 vaccination. HA-reactive B cells from the other infant maintained a more evenly distributed specificity. In year 2, class-switched HA-specific B cell IGHV somatic hypermutation (SHM) levels reached the average levels seen in adults. The HA-reactive plasma antibody repertoires of both infants comprised a relatively small number of antibody clonotypes, with one or two very abundant clonotypes. Thus, after the year 2 boost, both infants had overall B cell profiles that resembled those of adult controls. IMPORTANCE Influenza virus is a moving target for the immune system. Variants emerge that escape protection from antibodies elicited by a previously circulating variant ("antigenic drift"). The immune system usually responds to a drifted influenza virus by mutating existing antibodies rather than by producing entirely new ones. Thus, immune memory of the earliest influenza virus exposure has a major influence on later responses to infection or vaccination ("immune imprinting"). In the many studies of influenza immunity in adult subjects, imprinting has been from an early infection, since only in the past 2 decades have infants received influenza immunizations. The work reported in this paper is a pilot study of imprinting by the flu vaccine in two infants, who received the vaccine before experiencing an influenza virus infection. The results suggest that a quadrivalent (four-subtype) vaccine may provide an immune imprint less dominated by one subtype than does a monovalent infection.
Keywords: B cell memory; circulating antibodies; immune imprinting; influenza virus; viral immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fonville JM, Wilks SH, James SL, Fox A, Ventresca M, Aban M, Xue L, Jones TC, Le NMH, Pham QT, Tran ND, Wong Y, Mosterin A, Katzelnick LC, Labonte D, Le TT, van der Net G, Skepner E, Russell CA, Kaplan TD, Rimmelzwaan GF, Masurel N, de Jong JC, Palache A, Beyer WEP, Le QM, Nguyen TH, Wertheim HFL, Hurt AC, Osterhaus ADME, Barr IG, Fouchier RAM, Horby PW, Smith DJ. 2014. Antibody landscapes after influenza virus infection or vaccination. Science 346:996–1000. doi:10.1126/science.1256427. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
