The Paxillin MoPax1 Activates Mitogen-Activated Protein (MAP) Kinase Signaling Pathways and Autophagy through MAP Kinase Activator MoMka1 during Appressorium-Mediated Plant Infection by the Rice Blast Fungus Magnaporthe oryzae
- PMID: 36314807
- PMCID: PMC9765475
- DOI: 10.1128/mbio.02218-22
The Paxillin MoPax1 Activates Mitogen-Activated Protein (MAP) Kinase Signaling Pathways and Autophagy through MAP Kinase Activator MoMka1 during Appressorium-Mediated Plant Infection by the Rice Blast Fungus Magnaporthe oryzae
Abstract
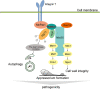
Paxillin is a focal adhesion-associated protein that functions as an adaptor to recruit diverse cytoskeleton and signaling molecules into a complex and plays a crucial role in several signaling pathways in mammal cells. However, paxillin-mediated signal pathways are largely unknown in phytopathogenic fungi. Previously, Pax1 of Magnaporthe oryzae (MoPax1), a paxillin-like protein, has been identified as a crucial pathogenicity determinant. Here, we report the identification of a mitogen-activated protein (MAP) kinase (MAPK) activator, Mka1 of M. oryzae (MoMka1), that physically interacts with MoPax1. Targeted gene deletion of MoMKA1 resulted in pleiotropic defects in aerial hyphal growth, conidiation, appressorium formation, and pathogenicity in M. oryzae. MoMka1 interacts with Mst50, an adaptor protein of the Mst11-Mst7-Pmk1 and Mck1-Mkk2-Mps1 cascades. Moreover, the phosphorylation levels of both Pmk1 and Mps1 in aerial hyphae of the ΔMomka1 mutant were significantly reduced, indicating that MoMka1 acts upstream from the MAPK pathways. Interestingly, we found that MoMka1 interacts with MoAtg6 and MoAtg13. Deletion of MoMKA1 led to impaired MoAtg13 phosphorylation and enhanced autophagic flux under nutrient-rich conditions, indicating that MoMka1 is required for regulation of autophagy in M. oryzae. Taken together, the paxillin MoPax1 may activate MAP kinase signaling pathways and autophagy through MAP kinase activator MoMka1 and play important roles during appressorium-mediated plant infection by the rice blast fungus. IMPORTANCE Paxillin, as an adaptor recruiting diverse cytoskeleton and signaling molecules into a complex, plays a crucial role in several signaling pathways in mammal cells. However, paxillin-mediated signal pathways are largely unknown in phytopathogenic fungi. Here, we identified that MoMka1 physically interacts with MoPax1. Furthermore, MoMka1 acts upstream from the MAPK pathways through interacting with Mst50, a key protein of the Mst11-Mst7-Pmk1 and Mck1-Mkk2-Mps1 cascades. Meanwhile, MoMka1 interacts with both MoAtg6 and MoAtg13 and controls autophagy initiation by influencing the phosphorylation level of MoAtg13. In summary, we describe a model in which MoPax1 activates MAP kinase signaling pathways and autophagy through MoMka1 during appressorium-mediated plant infection by M. oryzae.
Keywords: Magnaporthe oryzae; MoMka1; MoPax1; appressoria; autophagy; mitogen-activated protein kinases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
MST50 is involved in multiple MAP kinase signaling pathways in Magnaporthe oryzae.Environ Microbiol. 2017 May;19(5):1959-1974. doi: 10.1111/1462-2920.13710. Epub 2017 Apr 10. Environ Microbiol. 2017. PMID: 28244240
-
A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea.Plant Cell. 2005 Apr;17(4):1317-29. doi: 10.1105/tpc.104.029116. Epub 2005 Mar 4. Plant Cell. 2005. PMID: 15749760 Free PMC article.
-
Thioredoxins are involved in the activation of the PMK1 MAP kinase pathway during appressorium penetration and invasive growth in Magnaporthe oryzae.Environ Microbiol. 2016 Nov;18(11):3768-3784. doi: 10.1111/1462-2920.13315. Epub 2016 May 30. Environ Microbiol. 2016. PMID: 27059015
-
Mechanisms of regulated cell death during plant infection by the rice blast fungus Magnaporthe oryzae.Cell Death Differ. 2025 May;32(5):793-801. doi: 10.1038/s41418-024-01442-y. Epub 2025 Jan 10. Cell Death Differ. 2025. PMID: 39794451 Free PMC article. Review.
-
Under pressure: investigating the biology of plant infection by Magnaporthe oryzae.Nat Rev Microbiol. 2009 Mar;7(3):185-95. doi: 10.1038/nrmicro2032. Nat Rev Microbiol. 2009. PMID: 19219052 Review.
Cited by
-
Appressoria-Small but Incredibly Powerful Structures in Plant-Pathogen Interactions.Int J Mol Sci. 2023 Jan 21;24(3):2141. doi: 10.3390/ijms24032141. Int J Mol Sci. 2023. PMID: 36768468 Free PMC article. Review.
-
Pectate Lyase Genes Abundantly Expressed During the Infection Regulate Morphological Development of Colletotrichum camelliae and CcPEL16 Is Required for Full Virulence to Tea Plants.mSphere. 2023 Feb 21;8(1):e0067722. doi: 10.1128/msphere.00677-22. Epub 2023 Jan 24. mSphere. 2023. PMID: 36692304 Free PMC article.
-
An ULK1/2-PXN mechanotransduction pathway suppresses breast cancer cell migration.EMBO Rep. 2023 Nov 6;24(11):e56850. doi: 10.15252/embr.202356850. Epub 2023 Oct 17. EMBO Rep. 2023. PMID: 37846507 Free PMC article.
-
The Sordariomycetes: an expanding resource with Big Data for mining in evolutionary genomics and transcriptomics.Front Fungal Biol. 2023 Jun 30;4:1214537. doi: 10.3389/ffunb.2023.1214537. eCollection 2023. Front Fungal Biol. 2023. PMID: 37746130 Free PMC article. Review.
References
-
- De Jong JC, McCormack BJ, Smirnoff N, Talbot NJ. 1997. Glycerol generates turgor in rice blast. Nature 389:244–244. doi:10.1038/38418. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
