Safety and Pharmacokinetics of LHF-535, a Potential Treatment for Lassa Fever, in Healthy Adults
- PMID: 36314868
- PMCID: PMC9664847
- DOI: 10.1128/aac.00951-22
Safety and Pharmacokinetics of LHF-535, a Potential Treatment for Lassa Fever, in Healthy Adults
Abstract
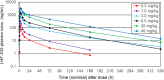
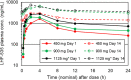
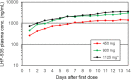
LHF-535 is a small-molecule antiviral currently under development as a therapeutic option to treat Lassa fever and other viral hemorrhagic fevers of arenavirus origin. The human safety and pharmacokinetics of LHF-535 were evaluated in two phase 1 trials in healthy volunteers. The first study was a double-blind, single ascending dose trial that evaluated weight-based oral doses ranging from 0.3 mg/kg in the first cohort to 40 mg/kg in the last cohort. The second study was a double-blind, multiple ascending dose trial that evaluated a 14-day oral dosing regimen, with three sequential cohorts receiving fixed doses of 450, 900, or 1,125 mg per day; the third cohort (1,125 mg/day) received a higher (loading) dose of 2,250 mg for the first dose. Each cohort in both studies consisted of eight participants randomized to either placebo (n = 2) or LHF-535 (n = 6). LHF-535 was well tolerated in both studies. Treatment-emergent adverse events were more frequent in placebo recipients than in LHF-535 recipients in both studies. LHF-535 exhibited rapid absorption, a long half-life, and exposures predicted to suppress viral replication.
Keywords: Lassa fever; antiviral agents; antiviral pharmacology.
Conflict of interest statement
The authors declare a conflict of interest. All Kineta authors (S.M.A., P.A.V.-G., E.J.T., J.P., K.M.B., and A.E.H.) are or were employees or consultants to Kineta, Inc., and may have some equity in the company. Funding was received from the Wellcome Trust.
Figures
References
-
- Grange ZL, Goldstein T, Johnson CK, Anthony S, Gilardi K, Daszak P, Olival KJ, O'Rourke T, Murray S, Olson SH, Togami E, Vidal G, Mazet JAK, Expert Panel, PREDICT Consortium . 2021. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc Natl Acad Sci USA 118:e2002324118. 10.1073/pnas.2002324118. - DOI - PMC - PubMed
-
- Akpede GO, Asogun DA, Okogbenin SA, Dawodu SO, Momoh MO, Dongo AE, Ike C, Tobin E, Akpede N, Ogbaini-Emovon E, Adewale AE, Ochei O, Onyeke F, Okonofua MO, Atafo RO, Odia I, Adomeh DI, Odigie G, Ogbeifun C, Muoebonam E, Ihekweazu C, Ramharter M, Colubri A, Sabeti PC, Happi CT, Günther S, Agbonlahor DE. 2019. Caseload and case fatality of Lassa fever in Nigeria, 2001–2018: a specialist center's experience and its implications. Front Public Health 7:170. 10.3389/fpubh.2019.00170. - DOI - PMC - PubMed
-
- Duvignaud A, Jaspard M, Etafo IC, Gabillard D, Serra B, Abejegah C, le Gal C, Abidoye AT, Doutchi M, Owhin S, Séri B, Vihundira JK, Bérerd-Camara M, Schaeffer J, Danet N, Augier A, Ogbaini-Emovon E, Salam AP, Ahmed LA, Duraffour S, Horby P, Günther S, Adedosu AN, Ayodeji OO, Anglaret X, Malvy D, LASCOPE Study Group . 2021. Lassa fever outcomes and prognostic factors in Nigeria (LASCOPE): a prospective cohort study. Lancet Glob Health 9:e469–e478. 10.1016/S2214-109X(20)30518-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

