Leveraging a Fluorescent Fatty Acid Probe to Discover Cell-Permeable Inhibitors of Plasmodium falciparum Glycerolipid Biosynthesis
- PMID: 36314974
- PMCID: PMC9769509
- DOI: 10.1128/spectrum.02456-22
Leveraging a Fluorescent Fatty Acid Probe to Discover Cell-Permeable Inhibitors of Plasmodium falciparum Glycerolipid Biosynthesis
Abstract
A sensitive and quantitative fluorescence-based approach is presented for characterizing fatty acid acquisition and lipid biosynthesis by asexually replicating, intraerythrocytic Plasmodium falciparum. We show that a BODIPY-containing, green-fluorescent fatty acid analog is efficiently and rapidly incorporated into parasite neutral lipids and phospholipids. Prelabeling with a red-fluorescent ceramide analog permits normalization and enables reliable quantitation of glycerolipid labeling. Inhibition of lipid labeling by competition with natural fatty acids and by acyl-coenzyme A synthetase and diacylglycerol acyltransferase inhibitors demonstrates that the fluorescent fatty acid probe is acquired, activated, and transferred to lipids through physiologically-relevant pathways. To assess its utility in discovering small molecules that block parasite lipid biosynthesis, the lipid labeling assay was used to screen a panel of mammalian lipase inhibitors and a selection of compounds from the "Malaria Box" anti-malarial collection. Several compounds were identified that inhibited the incorporation of the fluorescent fatty acid probe into lipids in cultured parasites at low micromolar concentrations. Two contrasting profiles of suppression of neutral lipid and phospholipid synthesis were observed, which implies the inhibition of distinct pathways. IMPORTANCE The human malaria parasite Plasmodium falciparum relies on fatty acid scavenging to supply this essential precursor of lipid synthesis during its asexual replication cycle in human erythrocytes. This dependence on host fatty acids represents a potential vulnerability that can be exploited to develop new anti-malarial therapies. The quantitative experimental approach described here provides a platform for simultaneously interrogating multiple facets of lipid metabolism- fatty acid uptake, fatty acyl-CoA synthesis, and neutral lipid and phospholipid biosynthesis- and of identifying cell-permeable inhibitors that are active in situ.
Keywords: Malaria Box; Plasmodium falciparum; fatty acid; fluorescent probe; malaria; neutral lipid; phospholipid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
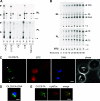

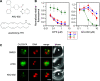

References
-
- WHO. 2021. World malaria report 2021.
-
- Brancucci NMB, Gerdt JP, Wang C, De Niz M, Philip N, Adapa SR, Zhang M, Hitz E, Niederwieser I, Boltryk SD, Laffitte MC, Clark MA, Gruring C, Ravel D, Blancke Soares A, Demas A, Bopp S, Rubio-Ruiz B, Conejo-Garcia A, Wirth DF, Gendaszewska-Darmach E, Duraisingh MT, Adams JH, Voss TS, Waters AP, Jiang RHY, Clardy J, Marti M. 2017. Lysophosphatidylcholine regulates sexual stage differentiation in the human malaria parasite Plasmodium falciparum. Cell 171:1532–1544.e15. doi:10.1016/j.cell.2017.10.020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

