Manganese-Enhanced Magnetic Resonance Imaging of the Heart
- PMID: 36314991
- PMCID: PMC10947173
- DOI: 10.1002/jmri.28499
Manganese-Enhanced Magnetic Resonance Imaging of the Heart
Abstract
Manganese-based contrast media were the first in vivo paramagnetic agents to be used in magnetic resonance imaging (MRI). The uniqueness of manganese lies in its biological function as a calcium channel analog, thus behaving as an intracellular contrast agent. Manganese ions are taken up by voltage-gated calcium channels in viable tissues, such as the liver, pancreas, kidneys, and heart, in response to active calcium-dependent cellular processes. Manganese-enhanced magnetic resonance imaging (MEMRI) has therefore been used as a surrogate marker for cellular calcium handling and interest in its potential clinical applications has recently re-emerged, especially in relation to assessing cellular viability and myocardial function. Calcium homeostasis is central to myocardial contraction and dysfunction of myocardial calcium handling is present in various cardiac pathologies. Recent studies have demonstrated that MEMRI can detect the presence of abnormal myocardial calcium handling in patients with myocardial infarction, providing clear demarcation between the infarcted and viable myocardium. Furthermore, it can provide more subtle assessments of abnormal myocardial calcium handling in patients with cardiomyopathies and being excluded from areas of nonviable cardiomyocytes and severe fibrosis. As such, MEMRI offers exciting potential to improve cardiac diagnoses and provide a noninvasive measure of myocardial function and contractility. This could be an invaluable tool for the assessment of both ischemic and nonischemic cardiomyopathies as well as providing a measure of functional myocardial recovery, an accurate prediction of disease progression and a method of monitoring treatment response. EVIDENCE LEVEL: 5: TECHNICAL EFFICACY: STAGE 5.
Keywords: calcium handling; kinetic modeling; manganese-enhanced magnetic resonance imaging.
© 2022 The Authors. Journal of Magnetic Resonance Imaging published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
None.
Figures

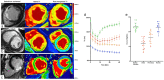
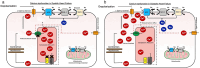
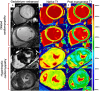
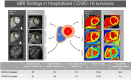
Similar articles
-
Manganese-enhanced MRI of the myocardium.Heart. 2019 Nov;105(22):1695-1700. doi: 10.1136/heartjnl-2019-315227. Epub 2019 Jul 23. Heart. 2019. PMID: 31337670 Free PMC article. Review.
-
Manganese-Enhanced T1 Mapping in the Myocardium of Normal and Infarcted Hearts.Contrast Media Mol Imaging. 2018 Oct 25;2018:9641527. doi: 10.1155/2018/9641527. eCollection 2018. Contrast Media Mol Imaging. 2018. PMID: 30498403 Free PMC article.
-
Assessment of stunned and viable myocardium using manganese-enhanced MRI.Open Heart. 2021 Jun;8(1):e001646. doi: 10.1136/openhrt-2021-001646. Open Heart. 2021. PMID: 34099530 Free PMC article.
-
Myocardial Viability Imaging using Manganese-Enhanced MRI in the First Hours after Myocardial Infarction.Adv Sci (Weinh). 2021 Jun;8(11):e2003987. doi: 10.1002/advs.202003987. Epub 2021 Apr 2. Adv Sci (Weinh). 2021. PMID: 34105284 Free PMC article.
-
Applications of Manganese-Enhanced Magnetic Resonance Imaging in Ophthalmology and Visual Neuroscience.Front Neural Circuits. 2019 May 14;13:35. doi: 10.3389/fncir.2019.00035. eCollection 2019. Front Neural Circuits. 2019. PMID: 31156399 Free PMC article. Review.
Cited by
-
Manganese-enhanced MRI during remotely induced myocardial ischemia reperfusion injury in male mice.Physiol Rep. 2025 Jul;13(13):e70442. doi: 10.14814/phy2.70442. Physiol Rep. 2025. PMID: 40605594 Free PMC article.
-
Manganese-Loaded Liposomes: An In Vitro Study for Possible Diagnostic Application.Molecules. 2024 Jul 20;29(14):3407. doi: 10.3390/molecules29143407. Molecules. 2024. PMID: 39064985 Free PMC article.
-
Non-invasive imaging of functional pancreatic islet beta-cell mass in people with type 1 diabetes mellitus.Diabet Med. 2023 Oct;40(10):e15111. doi: 10.1111/dme.15111. Epub 2023 Apr 21. Diabet Med. 2023. PMID: 37035965 Free PMC article.
References
-
- Lauterbur PCDM, Rudin AM. Augmentation of tissue water proton spin‐lattice relaxation rates by in vivo addition of paramagnetic ions. New York, NY: Academic Press; 1978.
Publication types
MeSH terms
Substances
Grants and funding
- FS/14/78/31020/BHF_/British Heart Foundation/United Kingdom
- CH/09/002/BHF_/British Heart Foundation/United Kingdom
- WT103782AIA/WT_/Wellcome Trust/United Kingdom
- FS/17/19/32641/BHF_/British Heart Foundation/United Kingdom
- FS/CRTF/20/24087/BHF_/British Heart Foundation/United Kingdom
- G0701127/MRC_/Medical Research Council/United Kingdom
- MR/T-29/53/1/MRC_/Medical Research Council/United Kingdom
- RE/18/5/34216/BHF_/British Heart Foundation/United Kingdom
- RG/16/10/32375/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- CH/09/002/26360/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Research Materials

